The collecting duct system of the kidney consists of a series of tubules and ducts that connect the nephrons to the ureter. It participates in electrolyte and fluid balance through reabsorption and excretion, processes regulated by the hormones aldosterone and antidiuretic hormone.
The collecting duct system of the kidney consists of the connecting tubule, the cortical collecting duct, and the medullary collecting duct. The collecting duct system is the final component of the kidney to influence the body's electrolyte and fluid balance. In humans, the system accounts for 4-5 percent of the kidney's reabsorption of sodium and 5% of the kidney's reabsorption of water. At times of extreme dehydration, over 24% of the filtered water may be reabsorbed in the collecting duct system.
The wide variation in water reabsorption levels for the collecting duct system reflects its dependence on hormonal activation. The collecting ducts, particularly the outer medullary and cortical collecting ducts, are largely impermeable to water without the presence of antidiuretic hormone (ADH, or vasopressin).
Monday, November 30, 2009
Sunday, November 29, 2009
Nephron
The nephron is the basic functional and structural unit of the kidney. Its main function is the purification and filtration of the blood. The nephron regulates the concentration of water and soluble substances like sodium salts by filtering the blood, reabsorbing what is needed and excreting the rest as urine. A nephron eliminates wastes from the body such as urea and creatinine, regulating blood volume and blood pressure, controlling levels of electrolytes and metabolites, and also blood pH. Its functions are vital to life and are regulated by hormones secreted by the endocrine system, such as antidiuretic hormone, aldosterone, and parathyroid hormone.

Tere are about one million nephrons in the cortex of each kidney, and each one consists of a renal corpuscle and a renal tubule which carry out the functions of the nephron. The renal tubule consists of the proximal convoluted tubule, the loop of Heinle, and distal convoluted tubule.
The nephron is part of the homeostatic mechanism of your body. This system helps regulate the amount of water, salts, glucose, urea and other minerals in your body. The nephron is a filtration system located in your kidney that is responsible for the reaborption of water, salts. This is where glucose eventually is absorbed in your body. One side note, diabetics have trouble reaborbing the glucose in their body and hence a lot of it comes out in the urine - hence the name "diabetic" or "sweet urine."
The Loop of Henle is the part of the nephron that contains the basic pathway for liquid. The liquid begins at the Bowman's capsule (upper left) and then flows through the proximal convoluted tubule (that mess of tangled stuff up top). It is here that Sodium, water, amino acids, and glucose get reabsorbed. The filtrate then flows down the descending limb and then back up. On the way it passes a major bend called the Loop Of Henle. This is located in the medulla of the kidney. As it approaches the top again, hydrogen ions (waste) flow into the tube and down the collecting duct. Essentially, nutrients flow in through the left and exit through the right. Along the way, salts, carbohydrates, and water pass through and are reabsorbed.

Friday, November 27, 2009
Podocytes
Podocytes, also known as visceral epithelial cells, are cells of the visceral epithelium in the kidneys, constituting a crucial component of the glomerular filtration barrier. The podocytes contribute in size selectivity and maintaining a massive filtration surface.

Podocytes are epithelial cells of the renal glomerulus, which are attached to the outer surface of the glomerular capillary basement membrane by cytoplasmic foot processes.
Podocytes

Thursday, November 26, 2009
Glomerular Basement Membrane
The glomerular basement membrane (GBM) is the basal laminal portion of the glomerulus which performs the actual filtration through the filtration slits between the podocytes, separating the blood on the inside from the filtrate on the outside. It is a fusion of the endothelial cell and podocyte basal laminas. The glomerular basement membrane is composed of three layers: 1) lamina rara externa, which consists of heparan sulfate and lies adjacent to podocyte processes; 2) lamina densa, which is situated in dark central zone and is composed of type 4 collagen and laminin; 3) lamina rara interna, which lies adjacent to endothelial cells and also consists of heparan sulfate.
The glomerular basement membrane forms the boundary between blood and urine. Across it, water and other small molecules from the blood are filtered. The GBM is composed of a meshwork of proteins and other constituents. Type IV collagen and laminin are present in the largest quantities. Some specialized subtypes of these molecules are only found in specialised basement membranes such as the GBM.

The glomerular basement membrane forms the boundary between blood and urine. Across it, water and other small molecules from the blood are filtered. The GBM is composed of a meshwork of proteins and other constituents. Type IV collagen and laminin are present in the largest quantities. Some specialized subtypes of these molecules are only found in specialised basement membranes such as the GBM.
Thin GBM disease
Thin glomerular basement membrane disease is the thinning of the basement membrane of the glomerulus. The GBM is thin, and must sometimes break, as it causes blood to appear in the urine. However it seems to repair without any ill effect, as thin GBM disease almost never causes serious trouble. The condition often runs in families, and can be a cause of benign familial haematuria.

Tuesday, November 24, 2009
Renal Corpuscle
The renal corpuscle, or Malpighian corpuscle, is the initial blood-filtering part of a kidney nephron. It is composed of the glomerulus and a Bowman's capsule. The glomerulus is a microscopic net of capillaries that contains two cell types: endothelial cells, which have large fenestrae; and mesangial cells, which are modified smooth muscle cells that lie between the capillaries and the glomerulus. These cells regulate blood flow by their contractile activity and secrete extracellular matrix, prostaglandins, and cytokines. The glomerulus is enclosed by the Bowman's capsule, which has an outer parietal layer composed of simple squamous epithelium and a visceral layer, which is composed of modified simple squamous epithelium lined by podocytes. Podocytes have foot processes, pedicels, that wrap around glomerular capillaries. These pedicels interdigitate with pedicels of adjacent podocytes forming filtration slits.

The renal corpuscle filtration barrier is composed of: the fenestrated endothelium of glomerular capillaries, the fused basal lamina of endothelial cells and podocytes, and the filtration slits of the podocytes. This barrier permits passage of water, ions, and small molecules from the bloodstream into Bowman's space (the space between the visceral and parietal layers). Large and/or negatively charged proteins are prevented from passing into Bowman's space, thus retaining these proteins in the circulation. The basal lamina is composed of 3 layers: lamina rara externa, lamina densa, and lamina rara interna. The lamina rara externa is adjacent to the podocyte processes. The lamina densa is the central layer consisting of type IV collagen and laminin. This layer acts as a selective macromolecular filter, preventing the passage of large protein molecules into Bowman's space. The lamina rara intena is adjacent to endothelial cells. This layer contains heparan sulfate, a negatively charged glycosaminoglycan that contributes to the electrostatic barrier of the glomerular filter.
There are two poles in the renal corpuscle, a vascular pole, and a urinary pole. The vascular pole is where the afferent and efferent arterioles communicate with the glomerulus. The urinary pole is where the corpuscle opens into the lumen of the proximal convoluted tubule.
Renal Corpuscle

Monday, November 23, 2009
Bowman's Capsule
The Bowman's capsule, or glomerular capsule, is a cup-like sac at the beginning of the renal tubule of a nephron in the kidney. The Bowman's capsule encloses and contains the glomerulus, which is the primary filtering device of the nephron. Blood is transported into the glomerulus contained in the Bowman's capsule from the afferent arteriole, which branches off of the interlobular artery. The cleaned blood exits at the vascular pole into the efferent arteriole, while the impurities flows into the renal tubule of the nephron and start working their way to the ureter.
When blood reaches the Bowman's capsule, it separates the blood into two components: a cleaned blood product, and a filtrate which is moved through the renal tubule of the nephron, another structure in the kidneys. As the filtrate travels along the renal tubule, additional impurities are removed, and the filtrate is concentrated into urine for the purpose of expressing waste products and excess water, which flow into collecting duct system.
Fom outside to inside, the Bowman's capsule is made up of the three layers: 1) parietal layer, which is a single layer of simple squamous epithelium; 2) visceral layer, which lies just beneath the thickened glomerular basement membrane and is made of podocytes (eneath the visceral layer lie the glomerular capillaries); 3) filtration Barrier, which is composed of the fenestrated endothelium of the glomerular capillaries, the fused basal lamina of the endothelial cells and podocytes, and the filtration slits of the podocytes. The barrier allows the passage of water, ions, and small molecules from the bloodstream into the Bowman's space. The barrier prevents the passage of large and/or negatively charged proteins, such as albumin.

When blood reaches the Bowman's capsule, it separates the blood into two components: a cleaned blood product, and a filtrate which is moved through the renal tubule of the nephron, another structure in the kidneys. As the filtrate travels along the renal tubule, additional impurities are removed, and the filtrate is concentrated into urine for the purpose of expressing waste products and excess water, which flow into collecting duct system.
Fom outside to inside, the Bowman's capsule is made up of the three layers: 1) parietal layer, which is a single layer of simple squamous epithelium; 2) visceral layer, which lies just beneath the thickened glomerular basement membrane and is made of podocytes (eneath the visceral layer lie the glomerular capillaries); 3) filtration Barrier, which is composed of the fenestrated endothelium of the glomerular capillaries, the fused basal lamina of the endothelial cells and podocytes, and the filtration slits of the podocytes. The barrier allows the passage of water, ions, and small molecules from the bloodstream into the Bowman's space. The barrier prevents the passage of large and/or negatively charged proteins, such as albumin.

Saturday, November 21, 2009
Glomerulus
The glomerulus is a tiny capillary network (twisted mass of microscopic tubes) which is located in the Bowman's capsule. Both the glomerulus and the Bowman's capsule make up the renal corpuscle of a nephron. The glomerulus is semipermeable; this means that it allows water and soluble wastes to seep through and be excreted out of the Bowman's capsule as urine. The filtered blood flows out of the glomerulus into the efferent arteriole to be returned through the medullary plexus to the intralobular vein.
Glomerulus and Bowman`s capsule



The glomerulus is the main filter of the nephron, and along with the Bowman's capsule, the basic filtration unit of the kidney. An afferent arteriole of the renal circulation drains into the glomerulus. In contrast with other capillary beds, the glomerulus drains into an efferent arteriole rather than a venule. The rate at which blood is filtered through all of the glomeruli in the kidney, and thus the measure of the overall renal function, is the glomerular filtration rate (GFR).
There are numerous pores, called fenestrae, in endothelial cells of the glomerulus. Unlike those of other fenestrated capillaries, are not spanned by diaphragms. These cells have openings which are so large that nearly anything smaller than a red blood cell passes through that layer. Because of this, the endothelial cells lining the glomerulus are not usually considered part of the renal filtration barrier. The glomerular endothelium sits on a very thick (250-350 nm) glomerular basement membrane. It is not only uncharacteristically thick compared to most other basement membranes (40-60 nm), but it is also rich in negatively charged glycosaminoglycans such as heparan sulfate.
Glomerulus and Bowman`s capsule



Friday, November 20, 2009
Renal Tubule
The renal tubule is the minute, long tubular partion of a nephron that leads away from the glomerulus. This minute reabsorptive tube of basement membrane is lined with epithelium, and compose the substance of the kidney. The renal tubule contains and conducts the tubular fluid which has been filtered through the glomerulus. After passing through the renal tubule, the filtrate continues to the collecting duct system, which is not part of the nephron. The renal tubule is made up of the proximal convoluted tubule, loop of Henle, and distal convoluted tubule, which empties into a collecting tubule.

The function of the renal tubule is reabsorption and secretion of useful and toxic materials respectively. Although the whole length of the renal tubule is involved in reabsorption, the cells of different regions of the renal tubule are adapted to perform specific transport functions, and consequently, the absorptive capacities of the different regions of the renal tubule differ.
Renal Tubule

Thursday, November 19, 2009
Proximal Convoluted Tubule
The proximal convoluted tubule is the section of the nephron which leads from Bowman's capsule to the loop of Henle. Coiled and lined with cells carpeted with microvilli and stuffed with mitochondria, the distinctive characteristic of the proximal tubule is its striated border.

The proximal tubule reabsorbs between 40 and 60% of the glomerular ultrafiltrate. Here, glucose and amino acids are reabsorbed in its totality along. The proximal convoluted tubule also reabsorbs 70% of the filtered potassium (K) and 75% of the bicarbonate (HCO3).
The cytoplasm of the cells that make up the proximal tubule is densely packed with mitochondria, which are largely found in the basal region within the infoldings of the basal plasma membrane. The high quantity of mitochondria gives the cells an acidophilic appearance. The high number of mitochondria is necessary to supply the energy for the active transport of sodium ions out of the proximal tubule. Water passively follows the sodium out of the cell along its concentration gradient.
The filtrate which accumulates in Bowman's space drains into the proximal tubule, and hence to the loop of Henle, the distal tubule, and the collecting duct. In these various segments of the renal tubule, the filtrate is modified into urine, chiefly by reabsorption of non-waste components.

Wednesday, November 18, 2009
Distal Convoluted Tubule
The distal convoluted tubule is the final portion of the nephron which is situated between the loop of Henle and the collecting duct system. The distal convoluted tubule is lined with cuboidal epithelial cells with abundant mitochondria and lateral membrane interdigitation with neighboring cells.
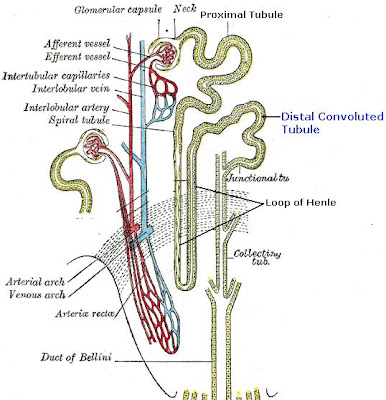
The distal convoluted tubule is mainly responsible for the reabsorption of water and sodium. It regulates pH through the absorption of bicarbonate and secretion of protons (H+) into the filtrate. Sodium absorption by the distal tubule is regulated by the hormone aldosterone. It also takes part in calcium regulation by secretion of excess Ca2+.
The distal tubule reabsorbs ~10% of the filtered Mg2+, but this is 70-80% of that delivered from the loop of Henle. Because there is little Mg2+ reabsorption beyond the distal tubule, this segment plays an important role in determining the final urinary excretion. The distal convoluted segment (DCT) is characterized by a negative luminal voltage and high intercellular resistance so that Mg2+ reabsorption is transcellular and active. This review discusses recent evidence for selective and sensitive control of Mg2+ transport in the DCT and emphasizes the importance of this control in normal and abnormal renal Mg2+ conservation.
Normally, Mg2+ absorption is load dependent in the distal tubule, whether delivery is altered by increasing luminal Mg2+ concentration or increasing the flow rate into the DCT. With the use of microfluorescent studies with an established mouse distal convoluted tubule (MDCT) cell line, it was shown that Mg2+ uptake was concentration and voltage dependent. Peptide hormones such as parathyroid hormone, calcitonin, glucagon, and arginine vasopressin enhance Mg2+ absorption in the distal tubule and stimulate Mg2+ uptake into MDCT cells. Prostaglandin E2 and isoproterenol increase Mg2+ entry into MDCT cells.
Distal Convoluted Tubule

Tuesday, November 17, 2009
Loop of Henle
The loop of Henle is the specialized portion of the nephron which leads from the proximal convoluted tubule to the distal convoluted tubule. The main function of the Henle's loop is to filter solutes. The ascending limb of the loop of Henle transports solutes, such as Sodium Chloride (NaCl), out of the tubule lumen with little or no water, generating an hyperosmotic medullary interstitium and delivering an hyposmotic tubule fluid to the distal tubule. Water present in the filtrate in the collecting duct flows through aquaporin channels out of the collecting duct, moving passively down its concentration gradient. This process reabsorbs water and creates a concentrated urine for excretion. This portion of the nephron was named after its discovery by the German physician Friedrich Gustav Jakob Henle.
The descending limb of the loop of Henle has low permeability to ions and urea, while being highly permeable to water. The thin ascending limb is not permeable to water, but it is permeable to ions. The medullary thick ascending limb remains impermeable to water with sodium, potassium (K+) and chloride (Cl-) ions being reabsorbed by active transport; K+ is passively transported along its concentration gradient through a K+ leak channel in the apical aspect of the cells, back into the lumen of the ascending limb. This K+ "leak" generates a positive electrochemical potential difference in the lumen. The electrical gradient drives more reabsorption of Na+, as well as other cations such as magnesium (Mg2+) and importantly calcium Ca2+.
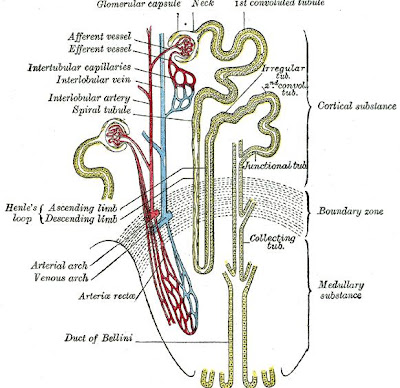
The descending limb of the loop of Henle has low permeability to ions and urea, while being highly permeable to water. The thin ascending limb is not permeable to water, but it is permeable to ions. The medullary thick ascending limb remains impermeable to water with sodium, potassium (K+) and chloride (Cl-) ions being reabsorbed by active transport; K+ is passively transported along its concentration gradient through a K+ leak channel in the apical aspect of the cells, back into the lumen of the ascending limb. This K+ "leak" generates a positive electrochemical potential difference in the lumen. The electrical gradient drives more reabsorption of Na+, as well as other cations such as magnesium (Mg2+) and importantly calcium Ca2+.
The loop of Henle is supplied by blood in a series of straight capillaries descending from the cortical efferent arterioles. These capillaries also have a countercurrent exchange mechanism that prevents washout of solutes from the medulla, thereby maintaining the medullary concentration. As water is osmotically driven from the descending limb into the interstitium, it readily enters the vasa recta. The low bloodflow through the vasa recta allows time for osmotic equilibration, and can be altered by changing the resistance of the vessels' efferent arterioles.

Sunday, November 15, 2009
Arcuate Nucleus
The arcuate nucleus is a group of neurons in the mediobasal hypothalamus, lying alongside the third ventricle and the median eminence. The arcuate nucleus includes several important populations of neurons, including: neuroendocrine neurons, which release dopamine into the hypophysial portal blood; and centrally-projecting neurons and Others.
The centrally-projecting neurons in the arcuate nucleus release neuropeptide Y, which influence hunger. When these neurons are activated, they can produce remarkable increases in eating that result in obesity. If such is the case, these neurons may be regulated by glucose, insulin, and the hormone leptin.
The centrally-projecting neurons in the arcuate nucleus release neuropeptide Y, which influence hunger. When these neurons are activated, they can produce remarkable increases in eating that result in obesity. If such is the case, these neurons may be regulated by glucose, insulin, and the hormone leptin.
Saturday, November 14, 2009
Neurohormone
A neurohormone is any hormone secreted and released by specialized neurons. A neurohormone is carried in the bloodstream to target cells. Neurohormones act as a messenger in the communication with other parts of the body; for example, the neurohormone ADH (antidiuretic hormone) is secreted in the pituitary gland and carried to the kidney, where it promotes water reabsorption in the kidney tubules. By contrast, neurotransmitters only serve local communication and may be considered paracrine hormones.
Other neurohormones are: thyrotropin-releasing hormone (TRH), Adrenocorticotropin-releasing hormone, Gonadotropin-releasing hormone (GnRH), and Oxytocin.
Other neurohormones are: thyrotropin-releasing hormone (TRH), Adrenocorticotropin-releasing hormone, Gonadotropin-releasing hormone (GnRH), and Oxytocin.
Friday, November 13, 2009
Supraoptic Nucleus
The supraoptic nucleus is a neuronal structure which lies in the hypothalamus alongside the optic chiasm at the base of the brain. It consists of magnocellular neurosecretory cells, which produce oxytocin and vasopressin hormones. These hormones are released in the general blood circulation by axons terminals in the supraoptic nucleus. In human, the supraoptic nucleus is made up of around 4,000 neurons.
Thursday, November 12, 2009
Magnocellular Neurosecretory Cells
Magnocellular neurosecretory cells are large neuroendocrine neurons which are found in the supraoptic nucleus and paraventricular nucleus of the hypothalamus. They are also found in smaller numbers in accessory cell groups between these two nuclei, the largest one being the nucleus circularis. Although there are two types of magnocellular neurosecretory cells, oxytocin-producing cells and vasopressin-producing cells, a small number can produce both hormones. These cells are neuroendocrine neurons, they are electrically excitable, and generate action potentials in response to afferent stimulation.
Magnocellular neurosecretory cells in the rat (where these neurons have been most extensively studied) generally have a single long varicose axon which projects to the posterior pituitary. Each axon gives rise to about 10,000 neurosecretory terminals and many axon swellings that store very large numbers of hormone-containing vesicles. These vesicles are released from the axon swellings and nerve terminals by exocytosis in response to calcium entry through voltage-gated channels, that occurs when action potentials are propagated down the axons.
The cells typically have two or three long dendrites, which also contain large dilations, and which also contain a very high density of hormone-containing vesicles. Oxytocin and vasopressin can thus be released within the brain from these dendrites, as well as into the blood from the terminals in the posterior pituitary gland. However the release of oxytocin and vasopressin from dendrites is not consistently accompanied by peripheral secretion, as dendritic release is regulated differently. Dendritic release can be triggered by depolarisation, but can also be triggered by the mobilization of intracellular calcium stores. The dendrites receive most of the synaptic inputs from afferent neurons that regulate the magnocellular neurons; typically a magnocellular neuron receives about 10,000 synapses from afferent neurons.
Magnocellular neurosecretory cells in the rat (where these neurons have been most extensively studied) generally have a single long varicose axon which projects to the posterior pituitary. Each axon gives rise to about 10,000 neurosecretory terminals and many axon swellings that store very large numbers of hormone-containing vesicles. These vesicles are released from the axon swellings and nerve terminals by exocytosis in response to calcium entry through voltage-gated channels, that occurs when action potentials are propagated down the axons.
The cells typically have two or three long dendrites, which also contain large dilations, and which also contain a very high density of hormone-containing vesicles. Oxytocin and vasopressin can thus be released within the brain from these dendrites, as well as into the blood from the terminals in the posterior pituitary gland. However the release of oxytocin and vasopressin from dendrites is not consistently accompanied by peripheral secretion, as dendritic release is regulated differently. Dendritic release can be triggered by depolarisation, but can also be triggered by the mobilization of intracellular calcium stores. The dendrites receive most of the synaptic inputs from afferent neurons that regulate the magnocellular neurons; typically a magnocellular neuron receives about 10,000 synapses from afferent neurons.
Neuroendocrine Neurons
Neuroendocrine neurons are group of specialized neurons which secrete dopamine, which is both a neurotransmitter and neurohormone. Neuroendocrine neurons are found in the arcuate nucleus of the hypothalamus. Their nerve endings release dopamine in the hypophysial portal blood. The dopamine is then carried in the bloodstream to the anterior lobe of the hypophysis (pituitary gland).
Neuroendocrine neurons are also called tuberoinfundibular dopamine neurons. In lactating females, the neuroendocrine neurons are inhibited by the stimulus of suckling. As dopamine inhibits prolactin secretion, there is increased secretion of prolactin when neuroendocrine neurons are inhibited.
Neuroendocrine neurons are also called tuberoinfundibular dopamine neurons. In lactating females, the neuroendocrine neurons are inhibited by the stimulus of suckling. As dopamine inhibits prolactin secretion, there is increased secretion of prolactin when neuroendocrine neurons are inhibited.
Wednesday, November 11, 2009
Circumventricular Organs
Circumventricular organs (CVO) are midline small structures situated at distinct sites around the margin of the ventricular system of the brain. They have common morphological and endocrine-like characteristics that distinguish them from the rest of the nervous system.
The circumventricular organs are among the few sites in the brain which have an incomplete blood-brain barrier. This means that neurons located in circumventricular organs can directly sense the concentrations of various compounds, particularly peptide hormones, in the bloodstream, without the need for specialized transport systems which move those compounds across the blood-brain barrier.
A useful mnemonic device for remembering this aspect of their function, though not the source of the name, is that they allow factors to 'circumvent' the blood-brain barrier. These organs secrete or are sites of action of a variety of different hormones, neurotransmitters and cytokines. They are sometimes classified by whether they are secretory or sensory.
The circumventricular organs are among the few sites in the brain which have an incomplete blood-brain barrier. This means that neurons located in circumventricular organs can directly sense the concentrations of various compounds, particularly peptide hormones, in the bloodstream, without the need for specialized transport systems which move those compounds across the blood-brain barrier.
A useful mnemonic device for remembering this aspect of their function, though not the source of the name, is that they allow factors to 'circumvent' the blood-brain barrier. These organs secrete or are sites of action of a variety of different hormones, neurotransmitters and cytokines. They are sometimes classified by whether they are secretory or sensory.
Tuesday, November 10, 2009
Paraventricular Nucleus
The paraventricular nucleus, or PVN, is a group of neurons in the hypothalamus. It is associated with the rear lobe of the pituitary gland. Within the paraventricular nucleus there are multiple subpopulations of neurons which are activated by a variety of physiological changes. About 40% of paraventricular nucleus neurons project directly to the posterior lobe of the pituitary, releasing oxytocin or vasopressin into the general circulation. Other PVN neurons control various anterior pituitary functions, while still others directly regulate appetite and autonomic functions in the brainstem and spinal cord.

The paraventricular nucleus lies alongside the third ventricle; hence its name, "paraventricular" meaning "alongside a ventricle." The PVN is highly vascularized and is protected by the blood-brain barrier, although its neuroendocrine neurons extend to sites beyond the blood-brain barrier.

Monday, November 9, 2009
Projections of the Retina
The projections of the retina are numerous, and all of the type of cells projecting to each central location have yet to be discovered. All vertebrates have the following central projections of the retina: 1) hypothalamus, 2) accesory optic system, 3) pretectum, 4) tectum, 5) ventral thalamus, dorsal thalamus. Since the pathways through both the tectum and dorsal thalamus eventually reach the visual cortex, they are considered to be in parallel. However, cells within either of these parallel pathways are connected to each other in a serial fashion.
Sunday, November 8, 2009
Prestriate Cortex
The prestriate cortex occupies the cortical surface area anterior, lateral, and superior to the striate cortex. Once believed to be cytoarchitectonicaly homogeneous, the prestriate cortex is actually a collection of about a dozen different areas. Much of this region used to be called the visual association area, because it did not receive direct inputs from the sensory thalamic relay nuclei.
In general, the amount of prestriate cortex represents a larger fraction of the visual area than the striate in higher mammals, while the reverse is true for lower species. This suggests that the more complex visual functions characteristic of primates may be associated with the prestriate cortex.
In general, the amount of prestriate cortex represents a larger fraction of the visual area than the striate in higher mammals, while the reverse is true for lower species. This suggests that the more complex visual functions characteristic of primates may be associated with the prestriate cortex.
Saturday, November 7, 2009
Pulvinar
The pulvinar is a caudal nucleus of the thalamus. The pulvinar complex is extensively and reciprocally interconnected in a visuotopic manner with many visual cortical areas. The pulvinar is usually divided into oral, inferior, lateral, and medial subnuclei. The lateral and inferior pulvinar are widely connected to visual cortical association areas; the oral pulvinar is linked with somatosensory cortical association areas; the medial pulvinar has widespread connections with cingulate, posterior parietal, and prefrontal cortical areas.
The number of fibers in the cortical, in contrast to the subcortical, input suggests that the response properties of pulvinar neurons may more mimic the former than the latter. Many cells are directionally and orientationally selective and have multiple discharge centers that are characteristic of prestriate but not tectal neurons.
The most dramatic differences between pulvinar and cortical neurons are receptive field size and breadth of orientation and directional tuning. Receptive field sizes of striate neurons are approximately 1º arc in width. Those of pulvinar cells average about 10º and may be as large as 60º. For the time being, the function of the pulvinar is uncertain. The coarse structure of the receptive field suggests that it does not analyze stimulus form.
The number of fibers in the cortical, in contrast to the subcortical, input suggests that the response properties of pulvinar neurons may more mimic the former than the latter. Many cells are directionally and orientationally selective and have multiple discharge centers that are characteristic of prestriate but not tectal neurons.
The most dramatic differences between pulvinar and cortical neurons are receptive field size and breadth of orientation and directional tuning. Receptive field sizes of striate neurons are approximately 1º arc in width. Those of pulvinar cells average about 10º and may be as large as 60º. For the time being, the function of the pulvinar is uncertain. The coarse structure of the receptive field suggests that it does not analyze stimulus form.
Friday, November 6, 2009
Median Eminence
The median eminence is the region of the brain which lies below the third cerebral ventricle and behind the optic chiasm. It is bounded laterally by the arcuate nucleus, rostral to the neural stalk that connects the posterior pituitary gland to the hypothalamus.
The median eminence consists of two zones, an internal zone and an external zone. The internal zone is made up of axons of the magnocellular neurosecretory neurons of the supraoptic nucleus and paraventricular nucleus which project to the posterior lobe of the pituitary gland. The external zone is composed of the convoluted blood vessels of the hypothalamo-pituitary portal system, and the nerve endings of many neuroendocrine neurons, such as the dopamine neurons of the arcuate nucleus and somatostatin neurons from the periventricular neucleus. These portal vessels transport releasing factors released by neuroendocrine neurones of the hypothalamus to the anterior pituitary gland.
The median eminence is of great physiological importance, as it is integral to the hypophyseal portal system, which connects the hypothalamus with the anterior lobe of the pituitary gland. It is in this structure that the secretions of the hypothalamus (releasing and inhibiting regulatory hormones) collect before entering the portal system.
The median eminence consists of two zones, an internal zone and an external zone. The internal zone is made up of axons of the magnocellular neurosecretory neurons of the supraoptic nucleus and paraventricular nucleus which project to the posterior lobe of the pituitary gland. The external zone is composed of the convoluted blood vessels of the hypothalamo-pituitary portal system, and the nerve endings of many neuroendocrine neurons, such as the dopamine neurons of the arcuate nucleus and somatostatin neurons from the periventricular neucleus. These portal vessels transport releasing factors released by neuroendocrine neurones of the hypothalamus to the anterior pituitary gland.
The median eminence is of great physiological importance, as it is integral to the hypophyseal portal system, which connects the hypothalamus with the anterior lobe of the pituitary gland. It is in this structure that the secretions of the hypothalamus (releasing and inhibiting regulatory hormones) collect before entering the portal system.
Wednesday, November 4, 2009
Inferior Parietal Lobule
The inferior parietal lobule is a cortical area in the parietal lobe of the brain. It lies behind the postcentral gyrus and below the superior parietal lobule. The inferior parietal lobule consists of two gyri: 1) the supramarginal gyrus, which arches over the upturned end ot the lateral fissure; 2) the angular gyrus, arching over the upper end of the superior temporal sulcus.

The inferior parietal lobule projects fibers to the middle and inferior frontal gyri and vice versa. After considerable research, it has been found that the inferior parietal lobule is involved in decision making under uncertainty, showing higher activity when the decision was uncertain rather than certain and when humans were given trial-by-trial feedback on choice outcomes than when they were not.
Inferior Parietal Lobule

Tuesday, November 3, 2009
Superior Parietal Lobule
The superior parietal lobule is an area of the cerebral cortex located in the parietal lobe, lying posteriorly to the postcentral gyrus and above the interparietal sulcus, which separates it from the inferior parietal lobule.

The superior parietal lobule (SPL) seems to be involved in spatial orientation and the somatic perception of bimanual interaction with an external object probably by computing the spatial relationship between the two hands and a hand-held object.
Superior Parietal Lobule

Monday, November 2, 2009
Parvocellular Pathway
The parvocellular pathway is the part of the visual system specialized in transmitting fine-grain highly detailed information. The parvocellular pathway begins with the midget bipolar ganglion cells in the foveal area of the retina and ends within the parvocellular layer of the lateral geniculate nucleus. Conduction is slower than that of Magnocellular Pathway.
Sunday, November 1, 2009
Magnocellular Pathway
The magnocellular pathway is the part of the visual system specialized in transmitting coarse- grain information and information about movement. The magnocellular pathway begins with the parasol, magnocellular ganglion cells in the non-foveal region of the retina and ends within the magnocellular layer of the lateral geniculate nucleus (LGN), which is the primary processing center for visual information received from the retina of the eye. Has a high contrast grain than Parvocellular Pathway.
The magnocellular pathway of the visual system sends more information to the right hemisphere thant to the left hemisphere. It is fast-conducting and is also responsible for our perception of stimulus change, which includes motion, and is largely color-blind. A review of the neurophysiological literature suggests that the magnocellular pathway has adequate spatial-frequency and contrast sensitivity to perceive text under normal contrast conditions.
The magnocellular pathway of the visual system sends more information to the right hemisphere thant to the left hemisphere. It is fast-conducting and is also responsible for our perception of stimulus change, which includes motion, and is largely color-blind. A review of the neurophysiological literature suggests that the magnocellular pathway has adequate spatial-frequency and contrast sensitivity to perceive text under normal contrast conditions.
Subscribe to:
Posts (Atom)