Goodpasture’s syndrome is a disease which affects the kidneys and lungs. It is characterized by glomerulonephritis and hemorrhaging of the lungs. The Goodpasture's syndrome is also called anti-glomerular basement membrane disease, which consists of progressive kidney failure which develops in days to weeks along with lung disease with cough, shortness of breath, and blood in the sputum. Although many diseases can present with these symptoms, the name Goodpasture’s syndrome is usually reserved for the autoimmune disease triggered when the patient’s immune system attacks Goodpasture antigen (a type II hypersensitivity reaction), which is found in the kidney and lung, and in time, causing damage to these organs. The disease bears the name of the American pathologist Dr. Ernest Goodpasture, whose 1919 description is regarded as the first report on the existence of the condition.
People who suffer from Goodpasture syndrome may feel a burning sensation when urinating. Other symptoms include fatigue or listlessness, nausea, difficulty breathing, or paleness. These symptoms are followed by small amounts of blood and protein in the urine. Aside from the presence of an inherited component, other possible causes are exposure to certain chemicals, including hydrocarbon solvents and the weed killer Paraquat, and viral infections. In the cases arising out of breathing hydrocarbon solvents, the immune system may attack organs or tissues because it mistakes them for viruses or foreign chemicals. This immune system's faulty response provokes bleeding in the air sacs and inflammation in the kidney's filtering units.
Goodpasture’s syndrome responds well to treatment with corticosteroids and immunosuppressants. These drugs dampen the body's normal immune response and the patient may become more susceptible to infections. The concentration of anti-GBM antibodies in the blood may be reduced by apheresis to remove blood plasma and replace a portion of the plasma with an isotonic salt and protein solution. This course of treatment usually lasts between three and six months.
Wednesday, December 30, 2009
Tuesday, December 29, 2009
Polyuria
Polyuria is a condition characterized by the passage of large volumes of urine (at least 2.5 liters in 24 hours in adults), which results in profuse urination and urinary frequency. Polyuria is a fairly common symptom, which is often noticed when you have to get up to use the bathroom at night.
Polyuria often appears in conjunction with polydipsia (increased thirst), but it is also possible to have one without the other, and the latter may be a cause or an effect. Psychogenic polydipsia may lead to polyuria.
Polyuria is physiologically normal in some circumstances, such as cold diuresis, altitude diuresis, and after drinking large amounts of fluids.
The causes of polyuria are: diabetes mellitus; increase in fluid intake, especially water; diuretic drugs; diuretic foods (foods and beverages containing caffeine, such as chocolate, coffee, tea, and soft drinks; diabetes insipidus; psychogenic polydipsia; high doses of riboflavin (vitamin B2); high doses of vitamin C; cold diuresis.
Polyuria often appears in conjunction with polydipsia (increased thirst), but it is also possible to have one without the other, and the latter may be a cause or an effect. Psychogenic polydipsia may lead to polyuria.
Polyuria is physiologically normal in some circumstances, such as cold diuresis, altitude diuresis, and after drinking large amounts of fluids.
The causes of polyuria are: diabetes mellitus; increase in fluid intake, especially water; diuretic drugs; diuretic foods (foods and beverages containing caffeine, such as chocolate, coffee, tea, and soft drinks; diabetes insipidus; psychogenic polydipsia; high doses of riboflavin (vitamin B2); high doses of vitamin C; cold diuresis.
Monday, December 28, 2009
Nephritis
Nephritis is inflammation of one or both kidneys. When the kidneys inflame, they begin to excrete needed protein from the body into the urine stream (proteinuria). Although nephritis can sometimes be caused by infection, it is most commonly caused by autoimmune disorders, such as lupus, which affects the major organs. For example, those with lupus are at a much higher risk for developing nephritis. It can also be genetically inherited, but it is rare. Nephritis is the ninth highest cause of human death.
Nephritis is the most common cause of glomerular injury. It is a disturbance of the glomerular structure with inflammatory cell proliferation. This can lead to: reduced glomerular blood flow leading to reduced urine output (oliguria) & retention of waste products (uremia). As a result, there can also be leakage of red blood cells from damaged glomerulus (hematuria). Low renal blood flow activates the renin-angiotensin-aldosterone system (RAAS), which therefore causes fluid retention and mild hypertension.
There are four types of nephritis: glomerulonephritis, interstitial nephritis, pyelonephritis, lupus nephritis. Nephritis causes the body to retain water, because the kidneys do not function properly to excrete the excess of water. Water retention can further cause swelling of the feet, ankles, legs, and hands.
Nephritis is the most common cause of glomerular injury. It is a disturbance of the glomerular structure with inflammatory cell proliferation. This can lead to: reduced glomerular blood flow leading to reduced urine output (oliguria) & retention of waste products (uremia). As a result, there can also be leakage of red blood cells from damaged glomerulus (hematuria). Low renal blood flow activates the renin-angiotensin-aldosterone system (RAAS), which therefore causes fluid retention and mild hypertension.
There are four types of nephritis: glomerulonephritis, interstitial nephritis, pyelonephritis, lupus nephritis. Nephritis causes the body to retain water, because the kidneys do not function properly to excrete the excess of water. Water retention can further cause swelling of the feet, ankles, legs, and hands.
Sunday, December 27, 2009
Lupus Nephritis
Lupus nephritis is an inflammation of the kidney caused by systemic lupus erythematosus (SLE), a disease of the immune system. It is characterized by damage to the glomerulus and progressive loss of kidney function. Lupus nephritis is triggered when antibodies and complement build up in the kidneys and cause inflammation, damaging the glomeruli. It also causes nephrotic syndrome with excessive protein excretion and may progress rapidly to renal failure. Thus, nitrogen waste products build up in the bloodstream.
The signs and symptoms of Lupus nephritis are weight gain, high blood pressure, darker foamy urine or swelling around the eyes, legs, ankles or fingers. Lupus nephritis affects approximately 3 out of 10,000 people. In children with SLE, about half will have some form or degree of kidney involvement.
The signs and symptoms of Lupus nephritis are weight gain, high blood pressure, darker foamy urine or swelling around the eyes, legs, ankles or fingers. Lupus nephritis affects approximately 3 out of 10,000 people. In children with SLE, about half will have some form or degree of kidney involvement.
Saturday, December 26, 2009
Alport Syndrome
Alport syndrome is a genetic disease which is characterized by glomerulonephritis, hearing impairment or loss, and loss of vision. Sometimes there is presence of blood in the urine (hematuria). Alport syndrome is triggered by genetic mutations that affect the type IV collagen family of proteins, which is a major part of important tissue structures called basement membranes that are present in all tissues that include the kidney, inner ear, and eye. At least 1 in 5,000 people suffers from Alport syndrome in the United States, affecting boys more than girls, since 80% of the time the disease is passed on by a mutation on the X chromosome.
Alport Syndrome always affects the kidneys. The primary symptom is blood in the urine (hematuria), which is usually microscopic, meaning it can only be detected with a microscope or a urine dipstick. Alport Syndrome causes damage to the kidneys by the progressive formation of scar tissue in the normal kidney structures (glomeruli and tubules). As the kidneys filter proteins out of the blood, these molecules damage the filtering system or glomeruli because of the abnormal collagen makeup. This process is known as fibrosis and it eventually leads to kidney failure.
Alport syndrome was first identified in a British family by Dr. Cecil A. Alport in 1927.
Alport Syndrome always affects the kidneys. The primary symptom is blood in the urine (hematuria), which is usually microscopic, meaning it can only be detected with a microscope or a urine dipstick. Alport Syndrome causes damage to the kidneys by the progressive formation of scar tissue in the normal kidney structures (glomeruli and tubules). As the kidneys filter proteins out of the blood, these molecules damage the filtering system or glomeruli because of the abnormal collagen makeup. This process is known as fibrosis and it eventually leads to kidney failure.
Alport syndrome was first identified in a British family by Dr. Cecil A. Alport in 1927.
Friday, December 25, 2009
Glomerulonephritis
Glomerulonephritis is a type renal disease that is characterized by inflammation of the glomeruli as the kidney capacity for filtering toxic waste and fluid from blood becomes damaged. Although glomerulonephritis can be caused by specific problems with the body's immune system, the precise cause of glomerulonephritis is often unknown. It may present as a nephrotic syndrome, acute renal failure, or chronic renal failure. Primary causes are ones which are intrinsic to the kidney, while secondary causes are associated with certain infections caused by bacterial, viral or parasitic pathogens.
The kidneys can be severely damaged before any symptoms appear. Evidence of glomerulonephritis may include: 1) noticeable swelling of the face, hands, feet, and ankles; 2) fatigue; high blood pressure; 3) blood and protein in the urine, which is a sign of damage to the glomeruli.
The condition may develop quickly, with loss of kidney function occurring over weeks and months; this called rapidly progressive glomerulonephritis.
Acute glomerulonephritis is a specific set of kidney diseases in which an immunologic mechanism triggers inflammation and proliferation of glomerular tissue that can result in damage to the basement membrane, mesangium, or capillary endothelium.
The kidneys can be severely damaged before any symptoms appear. Evidence of glomerulonephritis may include: 1) noticeable swelling of the face, hands, feet, and ankles; 2) fatigue; high blood pressure; 3) blood and protein in the urine, which is a sign of damage to the glomeruli.
The condition may develop quickly, with loss of kidney function occurring over weeks and months; this called rapidly progressive glomerulonephritis.
Acute glomerulonephritis is a specific set of kidney diseases in which an immunologic mechanism triggers inflammation and proliferation of glomerular tissue that can result in damage to the basement membrane, mesangium, or capillary endothelium.
Thursday, December 24, 2009
Interstitial Nephritis
Interstitial nephritis is a form of nephritis which affects the interstitium of the kidneys surrounding the tubules of the nephrons. The spaces between the kidney tubules become inflamed and swollen. The interstitial nephritis can affect the kidneys' function, including their ability to filter waste, eventually ending in kidney failure. It can be either acute or chronic.
The acute interstitial nephritis begins suddenly and is often caused by side effects of certain drugs. This form of the disease may be more severe and more likely to lead to chronic or permanent kidney damage in elderly people.
Common causes of interstitial nephritis include infection, or reaction to medication like analgesic or antibiotics. Reaction to medications causes 71% to 92% of cases. Both acute and chronic interstitial nephritis can also be caused by a bacterial infection in the kidneys, known as pyelonephritis.
Symptoms of interstitial nephritis may include: blood in the urine, increased or decreased urine output, fever, swelling of the body, rash, mental status changes such as drowsiness and confusion, nausea and vomiting, blood in urine.
The acute interstitial nephritis begins suddenly and is often caused by side effects of certain drugs. This form of the disease may be more severe and more likely to lead to chronic or permanent kidney damage in elderly people.
Common causes of interstitial nephritis include infection, or reaction to medication like analgesic or antibiotics. Reaction to medications causes 71% to 92% of cases. Both acute and chronic interstitial nephritis can also be caused by a bacterial infection in the kidneys, known as pyelonephritis.
Symptoms of interstitial nephritis may include: blood in the urine, increased or decreased urine output, fever, swelling of the body, rash, mental status changes such as drowsiness and confusion, nausea and vomiting, blood in urine.
Wednesday, December 23, 2009
Pyelonephritis
Pyelonephritis is an infection of the pelvis of the kidney as a result of ascending urinary tract infection. If the infection is severe, the term "urosepsis" is used interchangeably. Pyelonephritis is treated with antibiotics. It is also be called pyelitis. Pyelonephritis can be either acute, or chronic. Acute uncomplicated pyelonephritis is the sudden development of kidney inflammation. Chronic pyelonephritis is a long-standing infection that does not go away.
The symptoms are: chills with shaking, severe abdominal pain, back pain, fever, fatigue.
The symptoms are: chills with shaking, severe abdominal pain, back pain, fever, fatigue.
Tuesday, December 22, 2009
Urine
Urine is biological liquid composed of water, sodium, body toxic waste such as creatinine that is secreted by the kidneys by a process called urination and excreted through the urethra. Cellular metabolism generates numerous waste compounds, many rich in nitrogen, that require elimination from the bloodstream. This waste is eventually expelled from the body in a process known as micturition, the primary method for excreting water-soluble chemicals from the body. These chemicals can be detected and analyzed by urinalysis. Amniotic fluid is closely related to urine, and can be analyzed by amniocentesis.

Most animals have excretory systems to eliminate toxic soluble wastes. Human beings have a urinary system, which is made up of the kidneys, ureters, urinary bladder, and urethra, which excretes the toxic soluble wastes. The kidneys extract the soluble wastes from the bloodstream, as well as excess water, sugars, and a variety of other compounds. Remaining fluid contains high concentrations of urea and other substances, including toxins. Urine flows through these structures: the kidney, ureter, bladder, and finally the urethra. Urine is produced by a process of filtration, reabsorption, and tubular secretion.
Urine is amber-yellowish to white in color and odorless. Urine is produced by a process of filtration, reabsorption, and tubular secretion of the nephron. Urine is composed of 95% water, urea, inorganic salts, uric acid, creatinine, ammonia, and broken-down blood pigments, including urochrome, which makes urine yellow, plus any unusual substances not reabsorbed into the blood. When the kidneys are malfunctioning blood, protein, and urinary casts can also be found in urine.

Monday, December 21, 2009
Creatinine Clearance
Creatinine clearance rate (CCr) is the volume of blood plasma that is cleared of creatinine per unit time and is a useful measure for approximating the glomerular filtration rate (GFR). The creatinine clearance test compares the level of creatinine in urine with the creatinine level in the blood. Both GFR and CCr may be accurately calculated by comparative measurements of substances in the blood and urine, or estimated by formulas using just a blood test result (eGFR and eCCr).
The results of the creatinine clearance rate tests are important in assessing the excretory function of the kidneys. For example, grading of chronic renal insufficiency and dosage of drugs that are primarily excreted via urine are based on GFR (or creatinine clearance).
Normal Creatinine Clearance
Men
Average: 120 ml/min/1.73 m2 (+/-25) or 175 Liters/dayRange: 97-137 ml/min/1.73 m2 (0.93-1.32 ml/sec/m2 IU)
Women
Average: 95 ml/min/1.73 m2 (+/-20) or 135 Liters/day Range: 88-128 ml/min/1.73 m2 (0.85-1.23 ml/sec/m2 IU)
The results of the creatinine clearance rate tests are important in assessing the excretory function of the kidneys. For example, grading of chronic renal insufficiency and dosage of drugs that are primarily excreted via urine are based on GFR (or creatinine clearance).
Normal Creatinine Clearance
Men
Average: 120 ml/min/1.73 m2 (+/-25) or 175 Liters/dayRange: 97-137 ml/min/1.73 m2 (0.93-1.32 ml/sec/m2 IU)
Women
Average: 95 ml/min/1.73 m2 (+/-20) or 135 Liters/day Range: 88-128 ml/min/1.73 m2 (0.85-1.23 ml/sec/m2 IU)
Sunday, December 20, 2009
Creatinine
Creatinine is a chemical waste product of creatine phosphate, which is a very important molecule for energy production in muscles. Approximately 2% of the body's creatine is converted to creatinine every day. Creatinine is carried in the bloodstream to the kidneys where it is filtered out of the blood and excreted in the urine.
Depending on muscle mass, creatinine is usually produced at a fairly constant rate by the body. It is a spontaneously formed cyclic derivative of creatine. The kidneys filter creatinine out of the blood, with no tubular reabsorption of it. If the filtering of the kidney is deficient, blood creatinine levels rise. Thus the levels of creatinine contained in the blood and urine may be used to calculate the creatinine clearance (CrCl), which reflects the glomerular filtration rate (GFR), which is a measurement of renal function. However, in cases of severe renal dysfunction, the creatinine clearance rate will be "overestimated" because the active secretion of creatinine will account for a larger fraction of the total creatinine cleared. Ketoacids, cimetidine and trimethoprim reduce creatinine tubular secretion and therefore increase the accuracy of the GFR estimate, particularly in severe renal dysfunction.
Measuring serum creatinine is a simple test and it is the most commonly used indicator of renal function.A rise in blood creatinine levels can be observed only with marked damage to functioning nephrons. Thus, this test is not suitable for detecting early stage kidney disease. A better estimation of kidney function is given by the creatinine clearance test. Creatinine clearance can be accurately calculated using serum creatinine concentration and some or all of the following variables: sex, age, weight, and race as suggested by the American Diabetes Association without a 24 hour urine collection. Normal levels of creatinine in the blood are approximately 0.6 to 1.2 milligrams (mg) per deciliter (dl) in adult males and 0.5 to 1.1 milligrams per deciliter in adult females.
Depending on muscle mass, creatinine is usually produced at a fairly constant rate by the body. It is a spontaneously formed cyclic derivative of creatine. The kidneys filter creatinine out of the blood, with no tubular reabsorption of it. If the filtering of the kidney is deficient, blood creatinine levels rise. Thus the levels of creatinine contained in the blood and urine may be used to calculate the creatinine clearance (CrCl), which reflects the glomerular filtration rate (GFR), which is a measurement of renal function. However, in cases of severe renal dysfunction, the creatinine clearance rate will be "overestimated" because the active secretion of creatinine will account for a larger fraction of the total creatinine cleared. Ketoacids, cimetidine and trimethoprim reduce creatinine tubular secretion and therefore increase the accuracy of the GFR estimate, particularly in severe renal dysfunction.
Measuring serum creatinine is a simple test and it is the most commonly used indicator of renal function.A rise in blood creatinine levels can be observed only with marked damage to functioning nephrons. Thus, this test is not suitable for detecting early stage kidney disease. A better estimation of kidney function is given by the creatinine clearance test. Creatinine clearance can be accurately calculated using serum creatinine concentration and some or all of the following variables: sex, age, weight, and race as suggested by the American Diabetes Association without a 24 hour urine collection. Normal levels of creatinine in the blood are approximately 0.6 to 1.2 milligrams (mg) per deciliter (dl) in adult males and 0.5 to 1.1 milligrams per deciliter in adult females.
Saturday, December 19, 2009
Creatine Phosphate
Creatine phosphate, or phosphocreatine (PCr), is a phosphorylated creatine molecule that acts as a rapidly mobilizable reserve of high-energy phosphates in skeletal muscle and brain: creatine phosphate can anaerobically donate a phosphate group to ADP to form adenosine triphosphate (ATP) during the first 2 to 7 seconds following an intense muscular or neuronal effort. On the converse, excess ATP can be used during a period of low effort to convert creatine to phosphocreatine. The reversible phosphorylation of creatine is catalyzed by several creatine kinases. The presence of creatine kinase (CK-MB, MB for muscle/brain) in plasma is indicative of tissue damage and is used in the diagnosis of myocardial infarction. The cell's ability to generate phosphocreatine from excess ATP during rest, as well as its use of phosphocreatine for quick regeneration of ATP during intense activity, provides a spatial and temporal buffer of ATP concentration. In other words, creatine phosphate acts as high-energy reserve in a coupled reaction; the energy given off from donating the phosphate group is used to regenerate the other compound - in this case, ATP. Creatine phosphate plays a particularly important role in tissues that have high, fluctuating energy demands such as muscle and brain.
Phosphocreatine is formed from parts of three amino acids: Arginine (Arg), Glycine (Gly), and Methionine (Met). It can be synthesized by formation of guanidinoacetate from Arg and Gly (in kidney) followed by methylation (S-adenosyl methionine, SAM is required) to creatine (in liver), and phosphorylation by creatine kinase (ATP is required) to phosphocreatine (in muscle); catabolism: hydrolysis to creatinine. Phosphocreatine is synthesized in the liver and transported to the muscle cells, via the bloodstream, for storage. Creatine phosphate shuttle help transport of high energy phosphate from mitochondria.

Phosphocreatine is formed from parts of three amino acids: Arginine (Arg), Glycine (Gly), and Methionine (Met). It can be synthesized by formation of guanidinoacetate from Arg and Gly (in kidney) followed by methylation (S-adenosyl methionine, SAM is required) to creatine (in liver), and phosphorylation by creatine kinase (ATP is required) to phosphocreatine (in muscle); catabolism: hydrolysis to creatinine. Phosphocreatine is synthesized in the liver and transported to the muscle cells, via the bloodstream, for storage. Creatine phosphate shuttle help transport of high energy phosphate from mitochondria.

Friday, December 18, 2009
Urinary System
The urinary system, or the genitourinary system, is the organ system that excretes, stores, and eliminates urine. The urinary system consists two kidneys, two ureters, the bladder, the urethra, and the penis in males. The nephron, which is an evolutionary modification of the nephridium, is the kidney's functional unit. Waste is filtered from the blood and collected as urine in each kidney. Urine leaves the kidneys by ureters, and collects in the bladder. The bladder can distend to store urine that eventually leaves through the urethra.
Urinary System
Urinary System
Thursday, December 17, 2009
Urinary Bladder
The urinary bladder is a muscular amd membranous sac which collects urine excreted by the kidneys prior to disposal by urination. The bladder is elastic and muscular and lies on the pelvic floor. Urine enters the bladder via the ureters and exits via the urethra.

The urinary bladder usually holds 500-650 mL of urine (a pint). When the level of urine reaches about 25% of this amount, pressure of the accumulating fluid stimulates nervous impulses that relax the external sphincter, a muscle that forms a dense band around the urethra at the base of the bladder.
The detrusor muscle is a layer of the urinary bladder wall made of smooth muscle fibers arranged in spiral, longitudinal, and circular bundles. The bladder is lined with a special epithelium composed of transitional cells. When the bladder is stretched, this signals the parasympathetic nervous system to contract the detrusor muscle. This encourages the bladder to expel urine through the urethra.

Wednesday, December 16, 2009
Urethra
The urethra is a tube which carries urine from the urinary bladder to the outside of the body. In males, the urethra travels through the penis, and carries semen as well as urine. In females, the urethra is shorter, measuring between 3 and 4 cm in length; it runs almost vertical and emerges above the vaginal opening. In males, the urethra is much longer and measure between 18 and 20 cm long, with wide and narrow sections. The external urethral sphincter is a striated muscle that allows voluntary control over urination.
Tuesday, December 15, 2009
Uronephrosis
Uronephrosis, also hydronephrosis, is the abonormal dilation of the kidney due to either a congenital or stone, tumour or stricture of the ureter. It is also distention of the renal pelvis and tubules with urine.
Monday, December 14, 2009
Renal Physiology
Renal physiology is the study of the function of the kidney. It comprises all functions of the kidney, including reabsorption of glucose, amino acids, and other small molecules; regulation of sodium, potassium, and other electrolytes; regulation of fluid balance and blood pressure; maintenance of acid-base balance; and the production of various hormones including erythropoietin and vitamin D.
Much of renal physiology is studied at the level of the nephron, which is the smallest functional unit of the kidney. Each nephron begins with a filtration component that filters blood entering the kidney. This filtrate then flows along the length of the nephron, which is a tubular structure lined by a single layer of specialized cells and surrounded by capillaries. The major functions of these lining cells are the reabsorption of water and small molecules from the filtrate into the blood, and the secretion of wastes from the blood into the urine.
Proper function of the kidney requires that it receives and adequately filters blood. This is performed at the microscopic level by many hundreds of thousands of filtration units called renal corpuscles, each of which is composed of a glomerulus and a Bowman's capsule. A global assessment of renal function is often ascertained by estimating the rate of filtration, called the glomerular filtration rate (GFR).
Much of renal physiology is studied at the level of the nephron, which is the smallest functional unit of the kidney. Each nephron begins with a filtration component that filters blood entering the kidney. This filtrate then flows along the length of the nephron, which is a tubular structure lined by a single layer of specialized cells and surrounded by capillaries. The major functions of these lining cells are the reabsorption of water and small molecules from the filtrate into the blood, and the secretion of wastes from the blood into the urine.
Proper function of the kidney requires that it receives and adequately filters blood. This is performed at the microscopic level by many hundreds of thousands of filtration units called renal corpuscles, each of which is composed of a glomerulus and a Bowman's capsule. A global assessment of renal function is often ascertained by estimating the rate of filtration, called the glomerular filtration rate (GFR).
Renal Function
Renal function is the degree of efficiency the kidneys filter blood. So, The terms “renal function” and “kidney function” mean the same thing. For many people with reduced kidney function, a kidney disease is also present and will get worse. Serious health problems occur when people have less than 25 percent of their kidney function. When kidney function drops below 10 to 15 percent, a person needs some form of renal replacement therapy—either blood-cleansing treatments called dialysis or a kidney transplant—to sustain life.
Renal function is an indication of the state of the kidney and its role in renal physiology. Glomerular filtration rate (GFR) describes the flow rate of filtered fluid through the kidney. Creatinine clearance rate (CCr) is the volume of blood plasma that is cleared of creatinine per unit time and is a useful measure for approximating the GFR. Both GFR and CCr may be accurately calculated by comparative measurements of substances in the blood and urine, or estimated by formulas using just a blood test result (eGFR and eCCr).
The results of these tests are important in assessing the excretory renal function of the kidneys. For example, grading of chronic renal insufficiency and dosage of drugs that are primarily excreted via urine are based on GFR (or creatinine clearance).
It is commonly believed to be the amount of liquid filtered out of the blood that gets processed by the kidneys. Physiologically, these quantities (volumetric blood flow and mass removal) are only related loosely.
Most doctors use the plasma concentrations of the waste substances of creatinine and urea, as well as electrolytes to determine renal function. These measures are adequate to determine whether a patient is suffering from kidney disease.
Unfortunately, blood urea nitrogen (BUN) and creatinine will not be raised above the normal range until 60% of total kidney function is lost. Hence, the more accurate Glomerular filtration rate or its approximation of the creatinine clearance are measured whenever renal disease is suspected or careful dosing of nephrotoxic drugs is required.
Another prognostic marker for kidney disease is Microalbuminuria; the measurement of small amounts of albumin in the urine that cannot be detected by urine dipstick methods.
Renal function is an indication of the state of the kidney and its role in renal physiology. Glomerular filtration rate (GFR) describes the flow rate of filtered fluid through the kidney. Creatinine clearance rate (CCr) is the volume of blood plasma that is cleared of creatinine per unit time and is a useful measure for approximating the GFR. Both GFR and CCr may be accurately calculated by comparative measurements of substances in the blood and urine, or estimated by formulas using just a blood test result (eGFR and eCCr).
The results of these tests are important in assessing the excretory renal function of the kidneys. For example, grading of chronic renal insufficiency and dosage of drugs that are primarily excreted via urine are based on GFR (or creatinine clearance).
It is commonly believed to be the amount of liquid filtered out of the blood that gets processed by the kidneys. Physiologically, these quantities (volumetric blood flow and mass removal) are only related loosely.
Most doctors use the plasma concentrations of the waste substances of creatinine and urea, as well as electrolytes to determine renal function. These measures are adequate to determine whether a patient is suffering from kidney disease.
Unfortunately, blood urea nitrogen (BUN) and creatinine will not be raised above the normal range until 60% of total kidney function is lost. Hence, the more accurate Glomerular filtration rate or its approximation of the creatinine clearance are measured whenever renal disease is suspected or careful dosing of nephrotoxic drugs is required.
Another prognostic marker for kidney disease is Microalbuminuria; the measurement of small amounts of albumin in the urine that cannot be detected by urine dipstick methods.
Sunday, December 13, 2009
Renal Pelvis
The renal pelvis is the funnel-like dilated area at the center of the kidney. It is proximal to the ureter.The renal pelvis is the point of convergence of two or three major calyces. Each renal papilla is surrounded by a branch of the renal pelvis called a calyx. The renal pelvis is lined with a mucous-membrane layer that is only a few cells thick.
The renal pelvis functions as a funnel for urine flowing to the ureter. It is the location of several kinds of kidney cancer.

The renal pelvis functions as a funnel for urine flowing to the ureter. It is the location of several kinds of kidney cancer.

Saturday, December 12, 2009
Renal Cortex
The renal cortex is the outer part of the kidney which is situated between the renal capsule and the renal medulla. In the adult, it forms a continuous smooth outer zone with a number of projections (cortical columns) that extend down between the pyramids. The renal cortex is composed mainly of the renal corpuscles and the proximal and distal convoluted tubules, except for parts of the loop of Henle which descend into the renal medulla. It also contains blood vessels and cortical collecting ducts. The renal cortex is the part of the kidney where ultrafiltration occurs.

 Renal Cortex Cross Section
Renal Cortex Cross Section
Renal Cortex

 Renal Cortex Cross Section
Renal Cortex Cross Section Friday, December 11, 2009
Perinephric Fat
The perinephric fat, or perirenal fat, is an accumulation of extraperitoneal fat that completely surrounds the kidney. Enclosing this fat is a membranous condensation of extraperitoneal fascia, the renal fascia.

The perinephric fat is an adipose structure between the renal fascia and renal capsule, and can be regarded as a part of the latter. A different structure, the pararenal fat, is the adipose tissue superficial to the renal fascia.
Perirenal Fat

Thursday, December 10, 2009
Renal Capsule
The renal capsule is a tough fibrous layer of connective tissue which wraps up each kidney and it is covered in a thick layer of perinephric adipose tissue. The renal capsule provides some protection from trauma and damage. The perinephric fat may be regarded as a part of the renal capsule, called the adipose capsule of kidney.
Renal Capsule

Renal Capsule

Wednesday, December 9, 2009
Renal Hilum
The renal hilum of the kidney is the recessed central fissure on the medial border through which arteries, veins and ureter enter. The medial border of the kidney is concave in the center and convex toward either extremity. It is directed forward and a little downward. Its central part presents a deep longitudinal fissure, bounded by prominent overhanging anterior and posterior lips. This fissure is named the hilum, or hilus, and transmits the vessels, nerves, and ureter. From anterior to posterior, the renal vein exits, the renal artery enters, and the renal pelvis exits the kidney. From frontal to lateral order, the tubes entering the hilum of kidney are renal vein, renal artery and renal pelvis. The renal hilum was formerly called renal hilus.
Renal Hilum (Hilus)

Renal Hilum (Hilus)

Tuesday, December 8, 2009
Urinary Casts
Urinary casts are microscopic cylindrical particles which are produced by the kidney and are present in the urine in certain disease states. They build in the distal convoluted tubule and collecting ducts of nephrons, then dislodge and pass into the urine, where they can be detected by microscopy. Urinary casts can tell your doctor whether your urine is healthy or abnormal.
 Hyaline casts
Hyaline casts
 Granular casts
Granular casts
 Waxy casts
Waxy casts
 Epithelial casts
Epithelial casts
Urinary casts are held together by the Tamm-Horsfall mucoprotein which is secreted by renal tubule cells, and sometimes also by albumin in conditions of proteinuria. Cast formation is pronounced in environments favoring protein denaturation and precipitation. Tamm-Horsfall protein is particularly susceptible to precipitation in these conditions.
Types of urinary casts include: 1) hyaline casts, which are cylindrical and clear, with a low refractive index and can be seen in normal individuals in dehydration states or after vigorous exercises; 2) granular casts, which result either from the breakdown of cellular casts or the inclusion of aggregates of plasma proteins and they are a sign of chronic renal disease; 3) fatty casts, which are formed by the breakdown of lipid-rich epithelial cells and may be present in various renal disorders, such as the high urinary protein nephrotic syndrome; 4) waxy casts, which are associated with severe, longstanding kidney disease such as renal failure; 5) epithelial casts, which are formed by adhesion of desquamated epithelial cells of the tubule lining and can be seen in acute tubular necrosis and toxic ingestion, such as from mercury, diethylene glycol, or salicylate; 6) red blood cell casts.
Types of Urinary Casts
 Hyaline casts
Hyaline casts Granular casts
Granular casts Waxy casts
Waxy casts Epithelial casts
Epithelial castsMonday, December 7, 2009
Red Blood Cell Casts
Red blood cell casts are urinary casts whose presense in the urine is always pathologic, and is strongly indicative of glomerular damage, which can occur in glomerulonephritis from various causes or vasculitis, including Wegener's granulomatosis, systemic lupus erythematosus, post-streptococcal glomerulonephritis or Goodpasture’s syndrome. They can also be associated with renal infarction and subacute bacterial endocarditis.
Some times red blood cells stick together and form red blood cell casts. Such casts are indicative of glomerulonephritis, with leakage of RBC's from glomeruli, or severe tubular damage. They are a yellowish-brown color and are generally cylindrical with sometimes ragged edges; their fragility makes inspection of a fresh sample necessary. They are usually associated with nephritic syndromes.
Some times red blood cells stick together and form red blood cell casts. Such casts are indicative of glomerulonephritis, with leakage of RBC's from glomeruli, or severe tubular damage. They are a yellowish-brown color and are generally cylindrical with sometimes ragged edges; their fragility makes inspection of a fresh sample necessary. They are usually associated with nephritic syndromes.
Sunday, December 6, 2009
Waxy Casts
Waxy casts are urinary cast which suggest the very low urine flow associated with severe, longstanding kidney disease such as renal failure. Waxy casts are nephrology homogeneous cylindrical structures which are seen in the urine, and correspond to the degenerated cellular casts, and are typical of long-term oliguria and tubule obstruction. Additionally, due to urine stasis and their formation in diseased, dilated ducts, these casts are significantly larger than hyaline casts. While cylindrical, they also possess a higher refractive index and are more rigid, demonstrating sharp edges, fractures, and broken-off ends. Waxy casts also fall under the umbrella of “broad” casts, a more general term to describe the wider cast product of a dilated duct.

Although Waxy casts have a smooth consistency, they are more refractile and easier to see compared to hyaline casts. They commonly have squared off ends, as if they were brittle and easily broken. Waxy casts are a sign of tubular injury of a more chronic nature than granular or cellular casts and are always of pathologic significance.

Saturday, December 5, 2009
Fatty Casts
Fatty casts are urinary casts formed by the breakdown of lipid-rich epithelial cells, these are hyaline casts with fat globule inclusions, yellowish-tan in color. If cholesterol or cholesterol esters are present, fatty casts are associated with the “Maltese cross” sign under polarized light. They can be present in various disorders, including the high urinary protein nephrotic syndrome, diabetic or lupus nephropathy, or larger-scale necrosis or epithelial cell death.
Fatty Casts

Fatty casts are identified by the presence of refractile lipid droplets. The background matrix of the cast may be hyaline or granular in nature. Often, they are seen in urines in which free lipid droplets are present as well. Fatty casts are the most common type seen in cat urines. Interpretation of the significance of "fatty" casts should be based on the character of the cast matrix, rather than on the lipid content.
Fatty Casts

Friday, December 4, 2009
Granular Casts
Granular casts are the second-most common type of cast and they are the result either from the breakdown of cellular casts or the inclusion of aggregates of plasma proteins or immunoglobulin light chains. Depending on the size of inclusions, granular casts can be classified as fine or coarse, though the distinction has no diagnostic significance. Their appearance is generally more cigar-shaped and of a higher refractive index than hyaline casts. While most often indicative of chronic renal disease, granular casts, as with hyaline casts, can also be seen for a short time following strenuous exercise.

When cellular casts remain in the nephron for some time before they are flushed into the bladder urine, the cells may degenerate to become coarsely granular casts, then a finely granular cast, and finally, a waxy cast. Granular and waxy casts are believed to derive from renal tubular cell casts. Broad casts are believed to emanate from damaged and dilated tubules and are therefore seen in end-stage chronic renal disease.
Granular casts type II are hyaline matrix casts filled with granules similar to cytoplasmic degeneration granules. There is a relation between the structure of these granules and the granular cytoplasm of the degenerating tubular cells. Although the causes of this degeneration are unknown, a proteinuria is a usual finding. A protein overload could be responsible for the granular degeneration of the tubular cells. The cytoplasm granulation could then be integrated in a cast as free granules, as cytoplasmic fragments, or as complete cells.
Granular casts type I are casts embedding cellular debris. This type I cast has a variable size granulation with a clumpy distribution. The leukocytes origin of the debris is suspected, but our attempt to stain these casts with the Naphtyl AS-D Chloro-acetate esterase gave deceiving results. We think that these casts are made of cellular debris of different kinds including degenerated leukocytes. The term "cellular debris cast" would be more appropriate and less confusing than the type I granular casts.
Granular casts are a sign of underlying kidney disease. However, they are nonspecific and may be found in people with many different kidney disorders.

Thursday, December 3, 2009
Hyaline Casts
Hyaline casts are the most common type of urinary casts. Formed in the absence of cells in the tubular lumen, hyaline casts are solidified Tamm-Horsfall mucoprotein secreted from the tubular epithelial cells of individual nephrons. They have a smooth texture and a refractive index very close to that of the surrounding fluid. Low urine flow, concentrated urine, or an acidic environment can contribute to the formation of hyaline casts, and, as such, they may be seen in normal individuals in dehydration or vigorous exercise.

They are very difficult to see in wet preparations of urine and must be distinguished from mucus strands. Generally, hyaline casts have parallel sides with clear margins and blunted ends, whereas mucus strands are irregular in size with irregular margins. Reduced lighting is essential to see hyaline casts in urine sediment preparations.
Hyaline casts are cylindrical and clear, with a low refractive index, so that they can easily be missed on cursory review under brightfield microscopy, or in an aged sample where dissolution has occurred. On the other hand, phase contrast microscopy leads to easier identification. Given the ubiquitous presence of Tamm-Horsfall protein, other cast types are formed via the inclusion or adhesion of other elements to the hyaline base.
A hyaline cast

Wednesday, December 2, 2009
Tamm-Horsfall Protein
The Tamm-Horsfall protein is a glycoprotein isolated from normal urine by Tamm and Horsfall in the early fifties. This protein is excreted by the thick ascending branch of the loop of Henle and the first part of the distal tubules. Normal daily excreted quantity ranges from 25 to 50 mg. The protein has a huge molecular weight of around 7 millions Daltons, with 25% to 40% of its weight being carbohydrates. This protein is the major fraction of the uromucoprotein.
The Tamm-Horsfall protein is a glycoprotein that in humans is encoded by the UMOD gene. This gene encodes uromodulin, the most abundant protein in normal urine. Its excretion in urine follows proteolytic cleavage of the ectodomain of its glycosyl phosphatidylinosital-anchored counterpart that is situated on the luminal cell surface of the loop of Henle. Uromodulin may act as a constitutive inhibitor of calcium crystallization in renal fluids. Excretion of uromodulin in urine may provide defense against urinary tract infections caused by uropathogenic bacteria. Defects in this gene are associated with the autosomal dominant renal disorders medullary cystic kidney disease-2 (MCKD2) and familial juvenile hyperuricemic nephropathy. These disorders are characterized by juvenile onset of hyperuricemia, gout, and progressive renal failure. While several transcript variants may exist for this gene, the full-length natures of only two have been described to date. These two represent the major variants of this gene and encode the same isoform.
The Tamm-Horsfall protein (THP) is a GPI-anchored glycoprotein. It is produced by the thick ascending limb of the loop of Henle of mammalian kidney. While the monomeric molecule has a MW of approximately 68 kD, it is physiologically present in a highly aggregated state in urine. When this protein is concentrated at low pH, it forms a gel. Tamm-Horsfall protein is the most abundant protein in mammalian urine. It is the matrix of urinary casts derived from the secretion of renal tubular cells.
This protein precipitates as a gel in a 0.58M NaCl solution, and redissolves in deionized water, or in an alkaline buffered solution. If albumine is added to a pure water solution of TH protein, the latter will precipitate and form a gel, taking the shape of the glassware used. Because of this property, casts are believed to dissolve readily in diluted or alkaline urine. Transposing an in-vitro experiment in a real physiological situation is sometimes hasardous. Other factors could stabilize the cast, so that a slight alkalisation (bacterial growth) of the urine does not necessarily mean cast dissolution.
Studies using THP deficient mice revealed that THP may have a role in regulatory physiology and actually participates in transporter function. A role in bacterial binding and sequestration is suggested by studies showing that E.coli expressing MS (mannose-sensitive) pili or fimbriae can be trapped by Tamm-Horsfall protein via its mannose-containing side chains. Tamm-Horsfall protein is part of the matrix in renal calculi but a role in kidney stone formation remains debatable. Antibodies to Tamm-Horsfall protein have been seen in various forms of nephritis (eg, Balkan nephropathy), however, it remains unclear whether there is any (patho-)physiologic relevance to these findings.
The Tamm-Horsfall protein is a glycoprotein that in humans is encoded by the UMOD gene. This gene encodes uromodulin, the most abundant protein in normal urine. Its excretion in urine follows proteolytic cleavage of the ectodomain of its glycosyl phosphatidylinosital-anchored counterpart that is situated on the luminal cell surface of the loop of Henle. Uromodulin may act as a constitutive inhibitor of calcium crystallization in renal fluids. Excretion of uromodulin in urine may provide defense against urinary tract infections caused by uropathogenic bacteria. Defects in this gene are associated with the autosomal dominant renal disorders medullary cystic kidney disease-2 (MCKD2) and familial juvenile hyperuricemic nephropathy. These disorders are characterized by juvenile onset of hyperuricemia, gout, and progressive renal failure. While several transcript variants may exist for this gene, the full-length natures of only two have been described to date. These two represent the major variants of this gene and encode the same isoform.
The Tamm-Horsfall protein (THP) is a GPI-anchored glycoprotein. It is produced by the thick ascending limb of the loop of Henle of mammalian kidney. While the monomeric molecule has a MW of approximately 68 kD, it is physiologically present in a highly aggregated state in urine. When this protein is concentrated at low pH, it forms a gel. Tamm-Horsfall protein is the most abundant protein in mammalian urine. It is the matrix of urinary casts derived from the secretion of renal tubular cells.
This protein precipitates as a gel in a 0.58M NaCl solution, and redissolves in deionized water, or in an alkaline buffered solution. If albumine is added to a pure water solution of TH protein, the latter will precipitate and form a gel, taking the shape of the glassware used. Because of this property, casts are believed to dissolve readily in diluted or alkaline urine. Transposing an in-vitro experiment in a real physiological situation is sometimes hasardous. Other factors could stabilize the cast, so that a slight alkalisation (bacterial growth) of the urine does not necessarily mean cast dissolution.
Studies using THP deficient mice revealed that THP may have a role in regulatory physiology and actually participates in transporter function. A role in bacterial binding and sequestration is suggested by studies showing that E.coli expressing MS (mannose-sensitive) pili or fimbriae can be trapped by Tamm-Horsfall protein via its mannose-containing side chains. Tamm-Horsfall protein is part of the matrix in renal calculi but a role in kidney stone formation remains debatable. Antibodies to Tamm-Horsfall protein have been seen in various forms of nephritis (eg, Balkan nephropathy), however, it remains unclear whether there is any (patho-)physiologic relevance to these findings.
Tuesday, December 1, 2009
Kidneys
The kidneys are bean-shaped excretory organs which lie behind the abdominal cavity, in a space called the retroperitoneum. There are two kidneys, one on each side of the spinal column. They are approximately at the vertebral level T12 to L3. The kidneys receive blood from the paired renal arteries, and drain into the paired renal veins. Each kidney excretes urine into a ureter, itself a paired structure that empties into the urinary bladder. For life to be maintained, at least one kidney must function properly.


The kidneys function is to filter wastes, such as urea, creatinine, and salts, from the blood and excrete them, along with water, as urine. The kidney has a bean-shaped structure, with concave and convex surfaces. The concave surface, the renal hilum, is the point at which the renal artery enters the organ, and the renal vein and ureter leave. The kidney is surrounded by tough fibrous tissue, the renal capsule, which is itself surrounded by perinephric fat, renal fascia and paranephric fat. The anterior (front) border of these tissues is the peritoneum, while the posterior (rear) border is the transversalis fascia.
The kidney is divided into two major structures: the renal cortex, which is superficial, and the renal medulla, which lies deep below the surface. Grossly, these structures take the shape of 8 to 18 cone-shaped renal lobes, each containing renal cortex surrounding a portion of medulla called a renal pyramid (of Malphigi). Between the renal pyramids are projections of cortex called renal columns of Bertin. Spanning the cortex and medulla are the nephrons, which are the basic functional structures of the kidney; they are microscopic tubules that filter the toxic wastes, salts and get rid of excess water; there about one million nephrons per kidney. The initial filtering portion of a nephron is the renal corpuscle, located in the cortex, which is followed by a renal tubule that passes from the cortex deep into the medullary pyramids. Part of the renal cortex, a medullary ray is a collection of renal tubules that drain into a single collecting duct.
The tip, or papilla, of each pyramid empties urine into a minor calyx, minor calyces empty into major calyces, and major calyces empty into the renal pelvis, which becomes the ureter.
The essential tissue composition of kidney is that of a gland with highly modified secretory units and highly specialized ducts (nephrons). Kidneys excrete urine, produced by modifying a filtrate of blood plasma. The fundamental unit of the kidney is the nephron, and the fundamental unit of the nephron is the glomerulus.
Kidneys


Monday, November 30, 2009
Collecting Duct system
The collecting duct system of the kidney consists of a series of tubules and ducts that connect the nephrons to the ureter. It participates in electrolyte and fluid balance through reabsorption and excretion, processes regulated by the hormones aldosterone and antidiuretic hormone.
The collecting duct system of the kidney consists of the connecting tubule, the cortical collecting duct, and the medullary collecting duct. The collecting duct system is the final component of the kidney to influence the body's electrolyte and fluid balance. In humans, the system accounts for 4-5 percent of the kidney's reabsorption of sodium and 5% of the kidney's reabsorption of water. At times of extreme dehydration, over 24% of the filtered water may be reabsorbed in the collecting duct system.
The wide variation in water reabsorption levels for the collecting duct system reflects its dependence on hormonal activation. The collecting ducts, particularly the outer medullary and cortical collecting ducts, are largely impermeable to water without the presence of antidiuretic hormone (ADH, or vasopressin).
The collecting duct system of the kidney consists of the connecting tubule, the cortical collecting duct, and the medullary collecting duct. The collecting duct system is the final component of the kidney to influence the body's electrolyte and fluid balance. In humans, the system accounts for 4-5 percent of the kidney's reabsorption of sodium and 5% of the kidney's reabsorption of water. At times of extreme dehydration, over 24% of the filtered water may be reabsorbed in the collecting duct system.
The wide variation in water reabsorption levels for the collecting duct system reflects its dependence on hormonal activation. The collecting ducts, particularly the outer medullary and cortical collecting ducts, are largely impermeable to water without the presence of antidiuretic hormone (ADH, or vasopressin).
Sunday, November 29, 2009
Nephron
The nephron is the basic functional and structural unit of the kidney. Its main function is the purification and filtration of the blood. The nephron regulates the concentration of water and soluble substances like sodium salts by filtering the blood, reabsorbing what is needed and excreting the rest as urine. A nephron eliminates wastes from the body such as urea and creatinine, regulating blood volume and blood pressure, controlling levels of electrolytes and metabolites, and also blood pH. Its functions are vital to life and are regulated by hormones secreted by the endocrine system, such as antidiuretic hormone, aldosterone, and parathyroid hormone.

Tere are about one million nephrons in the cortex of each kidney, and each one consists of a renal corpuscle and a renal tubule which carry out the functions of the nephron. The renal tubule consists of the proximal convoluted tubule, the loop of Heinle, and distal convoluted tubule.
The nephron is part of the homeostatic mechanism of your body. This system helps regulate the amount of water, salts, glucose, urea and other minerals in your body. The nephron is a filtration system located in your kidney that is responsible for the reaborption of water, salts. This is where glucose eventually is absorbed in your body. One side note, diabetics have trouble reaborbing the glucose in their body and hence a lot of it comes out in the urine - hence the name "diabetic" or "sweet urine."
The Loop of Henle is the part of the nephron that contains the basic pathway for liquid. The liquid begins at the Bowman's capsule (upper left) and then flows through the proximal convoluted tubule (that mess of tangled stuff up top). It is here that Sodium, water, amino acids, and glucose get reabsorbed. The filtrate then flows down the descending limb and then back up. On the way it passes a major bend called the Loop Of Henle. This is located in the medulla of the kidney. As it approaches the top again, hydrogen ions (waste) flow into the tube and down the collecting duct. Essentially, nutrients flow in through the left and exit through the right. Along the way, salts, carbohydrates, and water pass through and are reabsorbed.

Friday, November 27, 2009
Podocytes
Podocytes, also known as visceral epithelial cells, are cells of the visceral epithelium in the kidneys, constituting a crucial component of the glomerular filtration barrier. The podocytes contribute in size selectivity and maintaining a massive filtration surface.

Podocytes are epithelial cells of the renal glomerulus, which are attached to the outer surface of the glomerular capillary basement membrane by cytoplasmic foot processes.
Podocytes

Thursday, November 26, 2009
Glomerular Basement Membrane
The glomerular basement membrane (GBM) is the basal laminal portion of the glomerulus which performs the actual filtration through the filtration slits between the podocytes, separating the blood on the inside from the filtrate on the outside. It is a fusion of the endothelial cell and podocyte basal laminas. The glomerular basement membrane is composed of three layers: 1) lamina rara externa, which consists of heparan sulfate and lies adjacent to podocyte processes; 2) lamina densa, which is situated in dark central zone and is composed of type 4 collagen and laminin; 3) lamina rara interna, which lies adjacent to endothelial cells and also consists of heparan sulfate.
The glomerular basement membrane forms the boundary between blood and urine. Across it, water and other small molecules from the blood are filtered. The GBM is composed of a meshwork of proteins and other constituents. Type IV collagen and laminin are present in the largest quantities. Some specialized subtypes of these molecules are only found in specialised basement membranes such as the GBM.

The glomerular basement membrane forms the boundary between blood and urine. Across it, water and other small molecules from the blood are filtered. The GBM is composed of a meshwork of proteins and other constituents. Type IV collagen and laminin are present in the largest quantities. Some specialized subtypes of these molecules are only found in specialised basement membranes such as the GBM.
Thin GBM disease
Thin glomerular basement membrane disease is the thinning of the basement membrane of the glomerulus. The GBM is thin, and must sometimes break, as it causes blood to appear in the urine. However it seems to repair without any ill effect, as thin GBM disease almost never causes serious trouble. The condition often runs in families, and can be a cause of benign familial haematuria.

Tuesday, November 24, 2009
Renal Corpuscle
The renal corpuscle, or Malpighian corpuscle, is the initial blood-filtering part of a kidney nephron. It is composed of the glomerulus and a Bowman's capsule. The glomerulus is a microscopic net of capillaries that contains two cell types: endothelial cells, which have large fenestrae; and mesangial cells, which are modified smooth muscle cells that lie between the capillaries and the glomerulus. These cells regulate blood flow by their contractile activity and secrete extracellular matrix, prostaglandins, and cytokines. The glomerulus is enclosed by the Bowman's capsule, which has an outer parietal layer composed of simple squamous epithelium and a visceral layer, which is composed of modified simple squamous epithelium lined by podocytes. Podocytes have foot processes, pedicels, that wrap around glomerular capillaries. These pedicels interdigitate with pedicels of adjacent podocytes forming filtration slits.

The renal corpuscle filtration barrier is composed of: the fenestrated endothelium of glomerular capillaries, the fused basal lamina of endothelial cells and podocytes, and the filtration slits of the podocytes. This barrier permits passage of water, ions, and small molecules from the bloodstream into Bowman's space (the space between the visceral and parietal layers). Large and/or negatively charged proteins are prevented from passing into Bowman's space, thus retaining these proteins in the circulation. The basal lamina is composed of 3 layers: lamina rara externa, lamina densa, and lamina rara interna. The lamina rara externa is adjacent to the podocyte processes. The lamina densa is the central layer consisting of type IV collagen and laminin. This layer acts as a selective macromolecular filter, preventing the passage of large protein molecules into Bowman's space. The lamina rara intena is adjacent to endothelial cells. This layer contains heparan sulfate, a negatively charged glycosaminoglycan that contributes to the electrostatic barrier of the glomerular filter.
There are two poles in the renal corpuscle, a vascular pole, and a urinary pole. The vascular pole is where the afferent and efferent arterioles communicate with the glomerulus. The urinary pole is where the corpuscle opens into the lumen of the proximal convoluted tubule.
Renal Corpuscle

Monday, November 23, 2009
Bowman's Capsule
The Bowman's capsule, or glomerular capsule, is a cup-like sac at the beginning of the renal tubule of a nephron in the kidney. The Bowman's capsule encloses and contains the glomerulus, which is the primary filtering device of the nephron. Blood is transported into the glomerulus contained in the Bowman's capsule from the afferent arteriole, which branches off of the interlobular artery. The cleaned blood exits at the vascular pole into the efferent arteriole, while the impurities flows into the renal tubule of the nephron and start working their way to the ureter.
When blood reaches the Bowman's capsule, it separates the blood into two components: a cleaned blood product, and a filtrate which is moved through the renal tubule of the nephron, another structure in the kidneys. As the filtrate travels along the renal tubule, additional impurities are removed, and the filtrate is concentrated into urine for the purpose of expressing waste products and excess water, which flow into collecting duct system.
Fom outside to inside, the Bowman's capsule is made up of the three layers: 1) parietal layer, which is a single layer of simple squamous epithelium; 2) visceral layer, which lies just beneath the thickened glomerular basement membrane and is made of podocytes (eneath the visceral layer lie the glomerular capillaries); 3) filtration Barrier, which is composed of the fenestrated endothelium of the glomerular capillaries, the fused basal lamina of the endothelial cells and podocytes, and the filtration slits of the podocytes. The barrier allows the passage of water, ions, and small molecules from the bloodstream into the Bowman's space. The barrier prevents the passage of large and/or negatively charged proteins, such as albumin.

When blood reaches the Bowman's capsule, it separates the blood into two components: a cleaned blood product, and a filtrate which is moved through the renal tubule of the nephron, another structure in the kidneys. As the filtrate travels along the renal tubule, additional impurities are removed, and the filtrate is concentrated into urine for the purpose of expressing waste products and excess water, which flow into collecting duct system.
Fom outside to inside, the Bowman's capsule is made up of the three layers: 1) parietal layer, which is a single layer of simple squamous epithelium; 2) visceral layer, which lies just beneath the thickened glomerular basement membrane and is made of podocytes (eneath the visceral layer lie the glomerular capillaries); 3) filtration Barrier, which is composed of the fenestrated endothelium of the glomerular capillaries, the fused basal lamina of the endothelial cells and podocytes, and the filtration slits of the podocytes. The barrier allows the passage of water, ions, and small molecules from the bloodstream into the Bowman's space. The barrier prevents the passage of large and/or negatively charged proteins, such as albumin.

Saturday, November 21, 2009
Glomerulus
The glomerulus is a tiny capillary network (twisted mass of microscopic tubes) which is located in the Bowman's capsule. Both the glomerulus and the Bowman's capsule make up the renal corpuscle of a nephron. The glomerulus is semipermeable; this means that it allows water and soluble wastes to seep through and be excreted out of the Bowman's capsule as urine. The filtered blood flows out of the glomerulus into the efferent arteriole to be returned through the medullary plexus to the intralobular vein.
Glomerulus and Bowman`s capsule



The glomerulus is the main filter of the nephron, and along with the Bowman's capsule, the basic filtration unit of the kidney. An afferent arteriole of the renal circulation drains into the glomerulus. In contrast with other capillary beds, the glomerulus drains into an efferent arteriole rather than a venule. The rate at which blood is filtered through all of the glomeruli in the kidney, and thus the measure of the overall renal function, is the glomerular filtration rate (GFR).
There are numerous pores, called fenestrae, in endothelial cells of the glomerulus. Unlike those of other fenestrated capillaries, are not spanned by diaphragms. These cells have openings which are so large that nearly anything smaller than a red blood cell passes through that layer. Because of this, the endothelial cells lining the glomerulus are not usually considered part of the renal filtration barrier. The glomerular endothelium sits on a very thick (250-350 nm) glomerular basement membrane. It is not only uncharacteristically thick compared to most other basement membranes (40-60 nm), but it is also rich in negatively charged glycosaminoglycans such as heparan sulfate.
Glomerulus and Bowman`s capsule



Friday, November 20, 2009
Renal Tubule
The renal tubule is the minute, long tubular partion of a nephron that leads away from the glomerulus. This minute reabsorptive tube of basement membrane is lined with epithelium, and compose the substance of the kidney. The renal tubule contains and conducts the tubular fluid which has been filtered through the glomerulus. After passing through the renal tubule, the filtrate continues to the collecting duct system, which is not part of the nephron. The renal tubule is made up of the proximal convoluted tubule, loop of Henle, and distal convoluted tubule, which empties into a collecting tubule.

The function of the renal tubule is reabsorption and secretion of useful and toxic materials respectively. Although the whole length of the renal tubule is involved in reabsorption, the cells of different regions of the renal tubule are adapted to perform specific transport functions, and consequently, the absorptive capacities of the different regions of the renal tubule differ.
Renal Tubule

Thursday, November 19, 2009
Proximal Convoluted Tubule
The proximal convoluted tubule is the section of the nephron which leads from Bowman's capsule to the loop of Henle. Coiled and lined with cells carpeted with microvilli and stuffed with mitochondria, the distinctive characteristic of the proximal tubule is its striated border.

The proximal tubule reabsorbs between 40 and 60% of the glomerular ultrafiltrate. Here, glucose and amino acids are reabsorbed in its totality along. The proximal convoluted tubule also reabsorbs 70% of the filtered potassium (K) and 75% of the bicarbonate (HCO3).
The cytoplasm of the cells that make up the proximal tubule is densely packed with mitochondria, which are largely found in the basal region within the infoldings of the basal plasma membrane. The high quantity of mitochondria gives the cells an acidophilic appearance. The high number of mitochondria is necessary to supply the energy for the active transport of sodium ions out of the proximal tubule. Water passively follows the sodium out of the cell along its concentration gradient.
The filtrate which accumulates in Bowman's space drains into the proximal tubule, and hence to the loop of Henle, the distal tubule, and the collecting duct. In these various segments of the renal tubule, the filtrate is modified into urine, chiefly by reabsorption of non-waste components.

Wednesday, November 18, 2009
Distal Convoluted Tubule
The distal convoluted tubule is the final portion of the nephron which is situated between the loop of Henle and the collecting duct system. The distal convoluted tubule is lined with cuboidal epithelial cells with abundant mitochondria and lateral membrane interdigitation with neighboring cells.
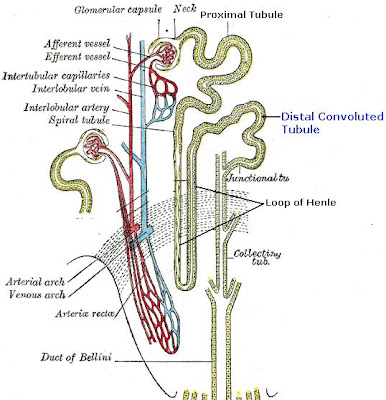
The distal convoluted tubule is mainly responsible for the reabsorption of water and sodium. It regulates pH through the absorption of bicarbonate and secretion of protons (H+) into the filtrate. Sodium absorption by the distal tubule is regulated by the hormone aldosterone. It also takes part in calcium regulation by secretion of excess Ca2+.
The distal tubule reabsorbs ~10% of the filtered Mg2+, but this is 70-80% of that delivered from the loop of Henle. Because there is little Mg2+ reabsorption beyond the distal tubule, this segment plays an important role in determining the final urinary excretion. The distal convoluted segment (DCT) is characterized by a negative luminal voltage and high intercellular resistance so that Mg2+ reabsorption is transcellular and active. This review discusses recent evidence for selective and sensitive control of Mg2+ transport in the DCT and emphasizes the importance of this control in normal and abnormal renal Mg2+ conservation.
Normally, Mg2+ absorption is load dependent in the distal tubule, whether delivery is altered by increasing luminal Mg2+ concentration or increasing the flow rate into the DCT. With the use of microfluorescent studies with an established mouse distal convoluted tubule (MDCT) cell line, it was shown that Mg2+ uptake was concentration and voltage dependent. Peptide hormones such as parathyroid hormone, calcitonin, glucagon, and arginine vasopressin enhance Mg2+ absorption in the distal tubule and stimulate Mg2+ uptake into MDCT cells. Prostaglandin E2 and isoproterenol increase Mg2+ entry into MDCT cells.
Distal Convoluted Tubule

Tuesday, November 17, 2009
Loop of Henle
The loop of Henle is the specialized portion of the nephron which leads from the proximal convoluted tubule to the distal convoluted tubule. The main function of the Henle's loop is to filter solutes. The ascending limb of the loop of Henle transports solutes, such as Sodium Chloride (NaCl), out of the tubule lumen with little or no water, generating an hyperosmotic medullary interstitium and delivering an hyposmotic tubule fluid to the distal tubule. Water present in the filtrate in the collecting duct flows through aquaporin channels out of the collecting duct, moving passively down its concentration gradient. This process reabsorbs water and creates a concentrated urine for excretion. This portion of the nephron was named after its discovery by the German physician Friedrich Gustav Jakob Henle.
The descending limb of the loop of Henle has low permeability to ions and urea, while being highly permeable to water. The thin ascending limb is not permeable to water, but it is permeable to ions. The medullary thick ascending limb remains impermeable to water with sodium, potassium (K+) and chloride (Cl-) ions being reabsorbed by active transport; K+ is passively transported along its concentration gradient through a K+ leak channel in the apical aspect of the cells, back into the lumen of the ascending limb. This K+ "leak" generates a positive electrochemical potential difference in the lumen. The electrical gradient drives more reabsorption of Na+, as well as other cations such as magnesium (Mg2+) and importantly calcium Ca2+.
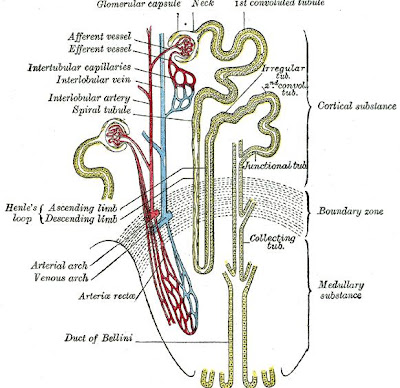
The descending limb of the loop of Henle has low permeability to ions and urea, while being highly permeable to water. The thin ascending limb is not permeable to water, but it is permeable to ions. The medullary thick ascending limb remains impermeable to water with sodium, potassium (K+) and chloride (Cl-) ions being reabsorbed by active transport; K+ is passively transported along its concentration gradient through a K+ leak channel in the apical aspect of the cells, back into the lumen of the ascending limb. This K+ "leak" generates a positive electrochemical potential difference in the lumen. The electrical gradient drives more reabsorption of Na+, as well as other cations such as magnesium (Mg2+) and importantly calcium Ca2+.
The loop of Henle is supplied by blood in a series of straight capillaries descending from the cortical efferent arterioles. These capillaries also have a countercurrent exchange mechanism that prevents washout of solutes from the medulla, thereby maintaining the medullary concentration. As water is osmotically driven from the descending limb into the interstitium, it readily enters the vasa recta. The low bloodflow through the vasa recta allows time for osmotic equilibration, and can be altered by changing the resistance of the vessels' efferent arterioles.

Sunday, November 15, 2009
Arcuate Nucleus
The arcuate nucleus is a group of neurons in the mediobasal hypothalamus, lying alongside the third ventricle and the median eminence. The arcuate nucleus includes several important populations of neurons, including: neuroendocrine neurons, which release dopamine into the hypophysial portal blood; and centrally-projecting neurons and Others.
The centrally-projecting neurons in the arcuate nucleus release neuropeptide Y, which influence hunger. When these neurons are activated, they can produce remarkable increases in eating that result in obesity. If such is the case, these neurons may be regulated by glucose, insulin, and the hormone leptin.
The centrally-projecting neurons in the arcuate nucleus release neuropeptide Y, which influence hunger. When these neurons are activated, they can produce remarkable increases in eating that result in obesity. If such is the case, these neurons may be regulated by glucose, insulin, and the hormone leptin.
Saturday, November 14, 2009
Neurohormone
A neurohormone is any hormone secreted and released by specialized neurons. A neurohormone is carried in the bloodstream to target cells. Neurohormones act as a messenger in the communication with other parts of the body; for example, the neurohormone ADH (antidiuretic hormone) is secreted in the pituitary gland and carried to the kidney, where it promotes water reabsorption in the kidney tubules. By contrast, neurotransmitters only serve local communication and may be considered paracrine hormones.
Other neurohormones are: thyrotropin-releasing hormone (TRH), Adrenocorticotropin-releasing hormone, Gonadotropin-releasing hormone (GnRH), and Oxytocin.
Other neurohormones are: thyrotropin-releasing hormone (TRH), Adrenocorticotropin-releasing hormone, Gonadotropin-releasing hormone (GnRH), and Oxytocin.
Friday, November 13, 2009
Supraoptic Nucleus
The supraoptic nucleus is a neuronal structure which lies in the hypothalamus alongside the optic chiasm at the base of the brain. It consists of magnocellular neurosecretory cells, which produce oxytocin and vasopressin hormones. These hormones are released in the general blood circulation by axons terminals in the supraoptic nucleus. In human, the supraoptic nucleus is made up of around 4,000 neurons.
Thursday, November 12, 2009
Magnocellular Neurosecretory Cells
Magnocellular neurosecretory cells are large neuroendocrine neurons which are found in the supraoptic nucleus and paraventricular nucleus of the hypothalamus. They are also found in smaller numbers in accessory cell groups between these two nuclei, the largest one being the nucleus circularis. Although there are two types of magnocellular neurosecretory cells, oxytocin-producing cells and vasopressin-producing cells, a small number can produce both hormones. These cells are neuroendocrine neurons, they are electrically excitable, and generate action potentials in response to afferent stimulation.
Magnocellular neurosecretory cells in the rat (where these neurons have been most extensively studied) generally have a single long varicose axon which projects to the posterior pituitary. Each axon gives rise to about 10,000 neurosecretory terminals and many axon swellings that store very large numbers of hormone-containing vesicles. These vesicles are released from the axon swellings and nerve terminals by exocytosis in response to calcium entry through voltage-gated channels, that occurs when action potentials are propagated down the axons.
The cells typically have two or three long dendrites, which also contain large dilations, and which also contain a very high density of hormone-containing vesicles. Oxytocin and vasopressin can thus be released within the brain from these dendrites, as well as into the blood from the terminals in the posterior pituitary gland. However the release of oxytocin and vasopressin from dendrites is not consistently accompanied by peripheral secretion, as dendritic release is regulated differently. Dendritic release can be triggered by depolarisation, but can also be triggered by the mobilization of intracellular calcium stores. The dendrites receive most of the synaptic inputs from afferent neurons that regulate the magnocellular neurons; typically a magnocellular neuron receives about 10,000 synapses from afferent neurons.
Magnocellular neurosecretory cells in the rat (where these neurons have been most extensively studied) generally have a single long varicose axon which projects to the posterior pituitary. Each axon gives rise to about 10,000 neurosecretory terminals and many axon swellings that store very large numbers of hormone-containing vesicles. These vesicles are released from the axon swellings and nerve terminals by exocytosis in response to calcium entry through voltage-gated channels, that occurs when action potentials are propagated down the axons.
The cells typically have two or three long dendrites, which also contain large dilations, and which also contain a very high density of hormone-containing vesicles. Oxytocin and vasopressin can thus be released within the brain from these dendrites, as well as into the blood from the terminals in the posterior pituitary gland. However the release of oxytocin and vasopressin from dendrites is not consistently accompanied by peripheral secretion, as dendritic release is regulated differently. Dendritic release can be triggered by depolarisation, but can also be triggered by the mobilization of intracellular calcium stores. The dendrites receive most of the synaptic inputs from afferent neurons that regulate the magnocellular neurons; typically a magnocellular neuron receives about 10,000 synapses from afferent neurons.
Neuroendocrine Neurons
Neuroendocrine neurons are group of specialized neurons which secrete dopamine, which is both a neurotransmitter and neurohormone. Neuroendocrine neurons are found in the arcuate nucleus of the hypothalamus. Their nerve endings release dopamine in the hypophysial portal blood. The dopamine is then carried in the bloodstream to the anterior lobe of the hypophysis (pituitary gland).
Neuroendocrine neurons are also called tuberoinfundibular dopamine neurons. In lactating females, the neuroendocrine neurons are inhibited by the stimulus of suckling. As dopamine inhibits prolactin secretion, there is increased secretion of prolactin when neuroendocrine neurons are inhibited.
Neuroendocrine neurons are also called tuberoinfundibular dopamine neurons. In lactating females, the neuroendocrine neurons are inhibited by the stimulus of suckling. As dopamine inhibits prolactin secretion, there is increased secretion of prolactin when neuroendocrine neurons are inhibited.
Wednesday, November 11, 2009
Circumventricular Organs
Circumventricular organs (CVO) are midline small structures situated at distinct sites around the margin of the ventricular system of the brain. They have common morphological and endocrine-like characteristics that distinguish them from the rest of the nervous system.
The circumventricular organs are among the few sites in the brain which have an incomplete blood-brain barrier. This means that neurons located in circumventricular organs can directly sense the concentrations of various compounds, particularly peptide hormones, in the bloodstream, without the need for specialized transport systems which move those compounds across the blood-brain barrier.
A useful mnemonic device for remembering this aspect of their function, though not the source of the name, is that they allow factors to 'circumvent' the blood-brain barrier. These organs secrete or are sites of action of a variety of different hormones, neurotransmitters and cytokines. They are sometimes classified by whether they are secretory or sensory.
The circumventricular organs are among the few sites in the brain which have an incomplete blood-brain barrier. This means that neurons located in circumventricular organs can directly sense the concentrations of various compounds, particularly peptide hormones, in the bloodstream, without the need for specialized transport systems which move those compounds across the blood-brain barrier.
A useful mnemonic device for remembering this aspect of their function, though not the source of the name, is that they allow factors to 'circumvent' the blood-brain barrier. These organs secrete or are sites of action of a variety of different hormones, neurotransmitters and cytokines. They are sometimes classified by whether they are secretory or sensory.
Tuesday, November 10, 2009
Paraventricular Nucleus
The paraventricular nucleus, or PVN, is a group of neurons in the hypothalamus. It is associated with the rear lobe of the pituitary gland. Within the paraventricular nucleus there are multiple subpopulations of neurons which are activated by a variety of physiological changes. About 40% of paraventricular nucleus neurons project directly to the posterior lobe of the pituitary, releasing oxytocin or vasopressin into the general circulation. Other PVN neurons control various anterior pituitary functions, while still others directly regulate appetite and autonomic functions in the brainstem and spinal cord.

The paraventricular nucleus lies alongside the third ventricle; hence its name, "paraventricular" meaning "alongside a ventricle." The PVN is highly vascularized and is protected by the blood-brain barrier, although its neuroendocrine neurons extend to sites beyond the blood-brain barrier.

Monday, November 9, 2009
Projections of the Retina
The projections of the retina are numerous, and all of the type of cells projecting to each central location have yet to be discovered. All vertebrates have the following central projections of the retina: 1) hypothalamus, 2) accesory optic system, 3) pretectum, 4) tectum, 5) ventral thalamus, dorsal thalamus. Since the pathways through both the tectum and dorsal thalamus eventually reach the visual cortex, they are considered to be in parallel. However, cells within either of these parallel pathways are connected to each other in a serial fashion.
Sunday, November 8, 2009
Prestriate Cortex
The prestriate cortex occupies the cortical surface area anterior, lateral, and superior to the striate cortex. Once believed to be cytoarchitectonicaly homogeneous, the prestriate cortex is actually a collection of about a dozen different areas. Much of this region used to be called the visual association area, because it did not receive direct inputs from the sensory thalamic relay nuclei.
In general, the amount of prestriate cortex represents a larger fraction of the visual area than the striate in higher mammals, while the reverse is true for lower species. This suggests that the more complex visual functions characteristic of primates may be associated with the prestriate cortex.
In general, the amount of prestriate cortex represents a larger fraction of the visual area than the striate in higher mammals, while the reverse is true for lower species. This suggests that the more complex visual functions characteristic of primates may be associated with the prestriate cortex.
Subscribe to:
Comments (Atom)
